Opportunities to Improve COVID-19 Vaccine Labels
Since the arrival of COVID-19 vaccines in Canada, information about their availability and use continues to be updated frequently. Recently, bivalent vaccines have been introduced to combat multiple strains of COVID-19 with a single vaccine dose. ISMP Canada has received numerous reports of incidents and concerns related to the labelling of these vaccines. In particular, the inability to differentiate the monovalent and bivalent vaccines resulted in errors of incorrect product and incorrect dose. This bulletin focuses on the labelling of these products, as a key factor contributing to the errors, and shares recommendations for both manufacturers and health care providers.
INTRODUCTION
Since the arrival of COVID-19 vaccines in Canada, information about their availability and use continues to be updated frequently. Recently, bivalent vaccines have been introduced to combat multiple strains of COVID-19 with a single vaccine dose. ISMP Canada has received numerous reports of incidents and concerns related to the labelling of these vaccines. In particular, the inability to differentiate the monovalent and bivalent vaccines resulted in errors of incorrect product and incorrect dose. This bulletin focuses on the labelling of these products, as a key factor contributing to the errors, and shares recommendations for both manufacturers and health care providers.
BACKGROUND
Given the public health emergency resulting from the current pandemic, Health Canada is permitting the use of COVID-19 vaccine products without Canadian-specific information (e.g., drug identification number [DIN]), to allow rapid, time-limited access to these products.1-4 Vaccine manufacturers are continuously updating labels to meet user needs and advance safety. As vaccine manufacturers plan to develop Canadian labels, there is an opportunity to guide good labelling practices by bringing together principles from the Good Label and Package Practices Guide for Prescription Drugs5 and learning from vaccine incidents.
DISCUSSION
Contributing factors and concerns from reported incidents related to labels circulating for the Moderna Spikevax bivalent vaccine for BA.1 (Figure 1B) and BA.4/5 (Figure 1C) at the time of the incidents included the following:
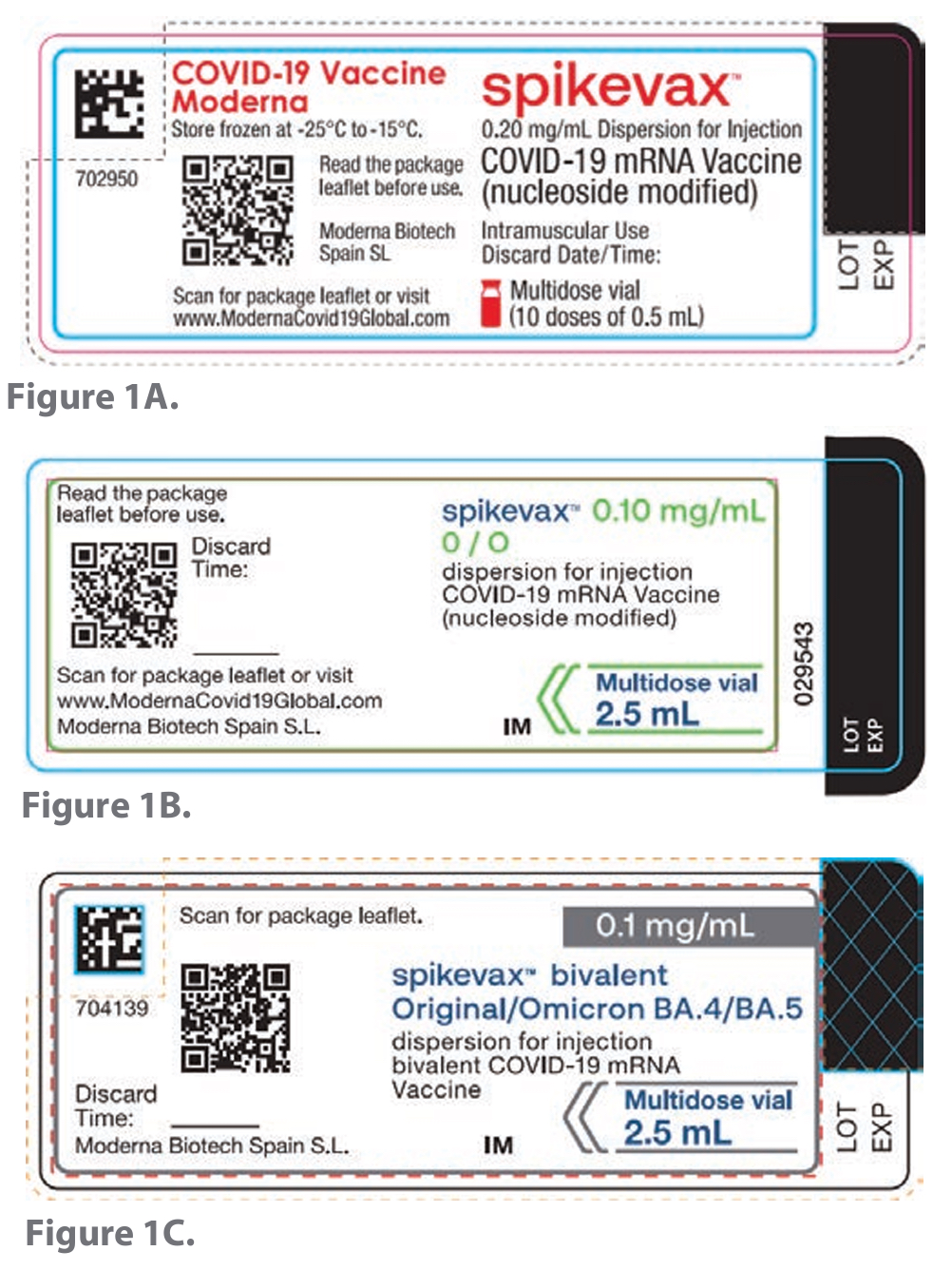
FIGURES 1A, 1B, AND 1C. Images of the Moderna Spikevax vaccine labels for adults. 1A represents a label for the monovalent product, while 1B and 1C depict labels of 2 different bivalent vaccines.
- A lack of distinctive, easy-to-understand descriptors, such as “bivalent” and “BA.1” on the product label in Figure 1B. For example, the potentially confusing designation “0/O” (intended to mean “zero/omicron”) appears on the label of 1 of the 2 circulating labels for the bivalent (BA.1) vaccine (Figure 1B).
- Absence of critical information from the vial label, such as the recommended dose in terms of volume.
- Inclusion of a package insert that did not contain usual product monograph information. Instead, the insert consisted of a folded page showing only “intentionally blank” and a data matrix code (Figure 2) that required downloading a data matrix scanner. In addition, when the code was scanned with the scanner, specific product information was not provided.
- Presence of a QR code on the vial label, which could be scanned by mobile devices to take the user to a webpage, however the user is required to self-select information for either the monovalent or bivalent vaccine.
- Inclusion of a trailing zero in the strength shown on multiple vaccine vial labels for adults (Figures 1A, 1B); this presentation is considered a dangerous dose designation.6
- Use of the same blue vial caps for both the vaccine for infants (6 months to 5 years of age; image not shared) and the bivalent vaccines for adults.

FIGURE 2. Image of a package insert without product information, but showing a data matrix code.
Contributing factors from reported incidents related to the labels of the Pfizer BioNTech Comirnaty bivalent vaccine (Figure 3B) included the following:
- The need to rotate the vial to view the word “bivalent” on the product label (Figure 3B).
- Look-alike packaging, with vials for both the monovalent and bivalent vaccines having a grey cap and white and grey labels (Figures 3A, 3B).
- Inconsistent use of dates on the label within the Comirnaty line of products. For example, the date on the COVID-19 vaccine products for children 5 to 11 years of age represents the date of manufacture, whereas the date on other products in this line represents the expiry date.7
- Misinterpretation of the company name BioNTech as “Bivalent”.

FIGURES 3A AND 3B. Images of Pfizer BioNTech monovalent (left) and bivalent (right) vaccines for adults.
RECOMMENDATIONS
Manufacturers
- Expedite the implementation of Canadian labels that meet the Food and Drug Regulations.
- Add or increase the prominence of the descriptors “bivalent” and specific virus variants on vial labels. For example, move the “bivalent” descriptor from the end of the first line on the Pfizer BioNTech product label so that critical information can be seen in one visual field.5
- Include a product insert that contains standard (expected) product information, such as dosing details. If a scannable code is included, ensure that it meets end-users’ needs and links to the specific information for the product in question, rather than necessitating that users choose from a menu of products.
- Add “Needs dilution” or “Do not dilute” to ensure that the directions for dose preparation are clear.
- Consider adding standardized automated identification (e.g., bar code) to facilitate product verification.
- Remove any unclear or dangerous abbreviations, symbols, or dose designations.
- Present dates consistently on vial labels.8 Practitioners are trained to interpret these dates as product expiry dates, not dates of manufacture.
- For products that will have a beyond-use date (i.e., last date/time that product can be used) differing from the product expiry date (e.g., after reconstitution), provide additional labels that can be affixed to the vial.
Practitioners
- Segregate the storage of each type of COVID-19 vaccine. For example, use labelled secondary containers (e.g., baskets), and ensure that look-alike products are stored in different areas of the fridge.9
- Consider administering only one type of vaccine (e.g., primary series or booster; adult or pediatric patients) on a given day or at a particular site.10
- Segregate the preparation and administration processes for each type of COVID-19 vaccine.10,11
- Display or make available resources (e.g., visual charts12) at the point of preparation/care outlining dose preparation and dosing information.9
CONCLUSION
Vaccines provide crucial protection against COVID-19 infection. Clear labelling of vaccines by manufacturers will reduce the likelihood of errors and optimize the safe use of these products.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
