A New Canadian Approach to High-Alert Medications
The bulletin describes the recent development of the Canadian High-Alert Medication List, as well as information about the accompanying user guide. This will support organizations in implementing an organization-specific list in their respective settings and aid accreditation processes.
Introduction
High-alert medications are medications that have an increased risk of causing significant patient harm when they are used in error.1 These medications are not necessarily more likely to be involved in an error or incident, but when such incidents occur, the consequences are often more severe relative to errors involving non–high-alert medications. The bulletin describes the recent development of the Canadian High-Alert Medication List, as well as information about the accompanying user guide. This will support organizations in implementing an organization-specific list in their respective settings and aid accreditation processes.
Download listThis Canadian list is applicable to all health care settings.
It is recommended that individual organizations adapt the list to reflect their local context.
Why Develop A Canadian High-Alert Medication List?
Historically, organizational lists of high-alert medications have been largely based on the lists developed by the US Institute for Safe Medication Practices.2-4 Other sources include the organizations’ own incident reporting systems, and reports, literature, and knowledge from medication safety experts. In response to interest expressed by and feedback received from Canadian practitioners, ISMP Canada has developed, with pan-Canadian collaboration, a Canadian High-Alert Medication List. This Canadian list importantly recognizes contextual differences between lists from other countries and seeks to affirm and validate currently recognized high-alert medications in the Canadian context.
A high-alert medication list supports the identification of drugs requiring additional safeguards to reduce the risk of errors and patient harm.5 Harm from these medications may be related to the intended treatment effects of the medication (e.g., reduction in serum glucose with insulin) or to adverse effects of the medication (e.g., respiratory depression with opioids).
This Canadian list is applicable to all health care settings. Given the range of health care sectors, the variety of care settings, the differing types of medications used, and the diversity of populations served, it is recommended that individual organizations adapt the list to reflect their local context. Targeted evidence-informed safety practices and incident data should also be taken into account in the development of an organization-specific organizational high-alert medication list.
Methodology
The Canadian High-Alert Medication List was developed through an extensive, multistep process (Figure 1).

FIGURE 1. Overview of the development process for the Canadian High-Alert Medication List.
Identification of Candidate Medications
Candidate medications for inclusion in the Canadian High-Alert Medication List were identified through 3 channels (Figure 2):
- Consultation with Canadian practitioners, stakeholders, patients, and caregivers.
- Analysis of incident reports associated with any level of harm from a recent 5-year period (January 1, 2018, to December 31, 2022). These reported incidents were extracted from 3 ISMP Canada reporting databases (Individual Practitioner Reporting, National Incident Data Repository for Community Pharmacies (NIDR), and Consumer Reporting, as well as from the National System for Incident Reporting (NSIR) database, administered by the Canadian Institute for Health Information (CIHI).
- Environmental scan of Canadian and international high-alert lists, medication safety reports, and literature.
This process yielded a candidate list of 79 drug classes and/or individual medications for potential inclusion on the Canadian High-Alert Medication List.
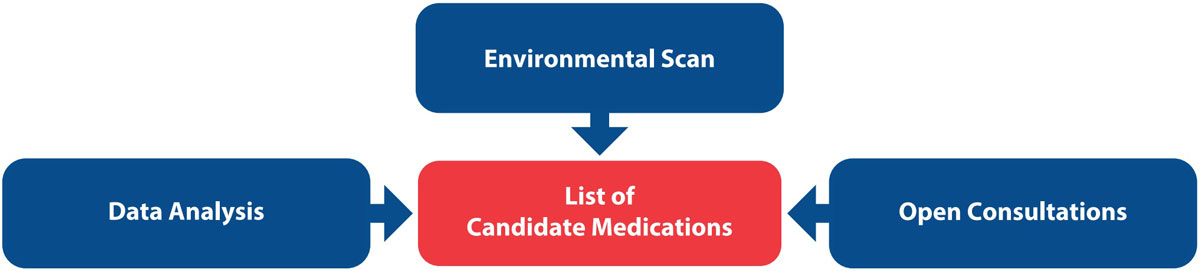
FIGURE 2. Information flow for identifying candidate medications.
Expert Opinions from Advisory Panel
A multisector, multidisciplinary advisory panel composed of practitioners from across Canada participated in an externally managed, 2-round Delphi process to provide guidance in developing the list. Panel members submitted their opinions regarding inclusion of candidate drug classes and/or individual medications on the proposed high-alert list and were also encouraged to suggest additional medications. In addition, the panel provided valuable guidance and feedback on the implications of designating a medication as “high-alert” and implementing the associated necessary safety processes.
Review by ISMP Canada
Members of the multidisciplinary ISMP Canada analysis team, assisted by internal medication safety specialists and leaders with clinical backgrounds, reviewed and finalized the list of candidate medications.
How to Use the Canadian High-Alert Medication List
The Canadian High-Alert Medication List is divided into medication classes, medications used in specific circumstances, and specific medications.
The taxonomy of the list is designed to reflect usage in typical care settings; it does not necessarily reflect any formal classification system (e.g., Anatomical Therapeutic Chemical classification or American Hospital Formulary System). Examples of each class or subclass are provided, but these are not meant to be inclusive. Two of the three formats available on the webpage provide a list of safety considerations and example risks associated with each class or subclass of medications.
The development process for an organization-specific list will be informed by evidence available through local incident reporting, local safety committees, and staff feedback, as well as knowledge gained from medication safety organizations. Following this thoughtful process, it is possible that some of the medications on the Canadian High-Alert Medication List may not appear on an individual organization’s list. Organizations may identify medications that warrant inclusion on their organization-specific high-alert list because of potential vulnerabilities recognized during their risk assessment. In this latter scenario, organizations are encouraged to share with ISMP Canada any medications or medication classes included on the organization-specific list that are not on the Canadian High-Alert Medication List, to inform future revisions and continuous improvement.
ISMP Canada has developed a user guide to assist organizations in establishing and implementing their high-alert medication lists and associated safety processes, and meeting accreditation requirements.6 The Canadian High-Alert Medication List (in the three formatted options) and user guide are available here: https://ismpcanada.ca/highalertlist
The Canadian High-Alert Medication List is intended to assist care organizations in developing their own organization-specific high-alert medication list, based upon a review and/or risk assessment of medications in use, as well as the population(s) served. This Canadian list is not intended to be adopted in its entirety as an organization-specific list.
Recommendations for Health Care Organizations
- Use the Canadian High-Alert Medication List to assist in establishing an organization-specific high-alert medication list, based upon a review and/or risk assessment of medications in use, as well as the population(s) served.
- Develop and implement safety strategies that reduce the risk of harm for each of the classes or individual medications on the organization-specific high-alert medication list, keeping the following principles in mind:
- Safety strategies should be multiple and layered, reflecting effective interventions according to the hierarchy of effectiveness (see example in Figure 3).5,7
- Strategies ideally should address the underlying cause of any known incidents.5
- Independent double checks should be used in conjunction with other strategies, not as the sole method of verifying medications. They should be implemented at the point(s) in the medication-use process where they will have the strongest impact.
- Continuously monitor incident data and practitioner feedback to identify opportunities and take action to improve the organization-specific list, as well as associated safety strategies.
- Periodically review the organization-specific list to identify any medications that may warrant inclusion or removal to reflect changes in practices or formularies over time.
- Share with ISMP Canada any additional medications, classes, or situations included on the organization-specific list to inform updates to the Canadian High-Alert Medication List.
- Grow staff awareness of the organization-specific list and the accompanying risk-reduction strategies.
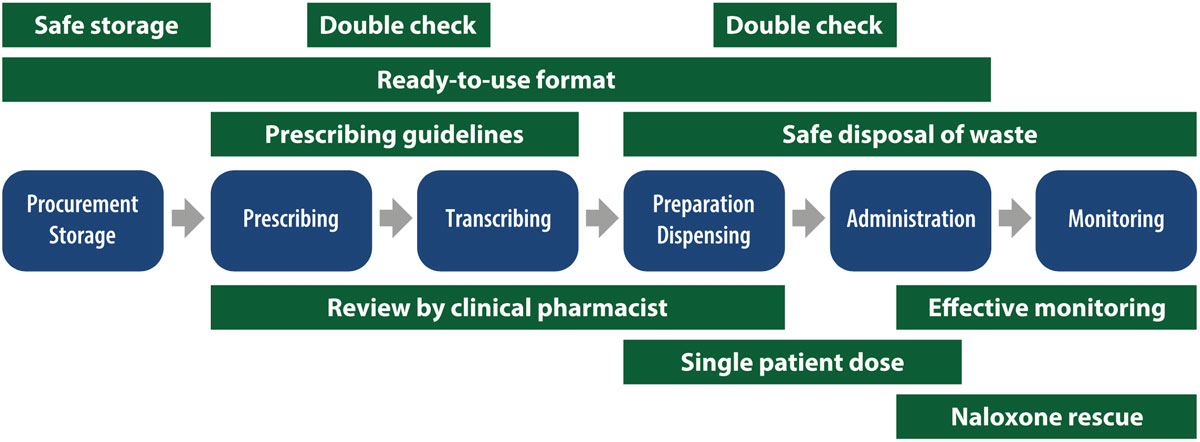
Figure 3. Example of multiple overlapping safety strategies used in the medication use process of opioid medications.
Conclusion
ISMP Canada developed the evidence-informed Canadian High-Alert Medication List through a multifactorial, multi-input process, with contributions from patients, caregivers and practitioners from many sectors and regions across Canada. The list is designed to be used as the basis for establishing organization-specific high-alert medication lists according to the local practice context and local factors of individual health care organizations. To reduce the risk of harm to patients, each organization should ensure that multiple, layered, and effective interventions are built into the management of medications on its organization-specific list. ISMP Canada will periodically update the Canadian High-Alert Medication List as new practice patterns, new technologies, new medications, and emerging issues are identified through reporting and shared learning and experiences.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.

