Introduction
Although safe in recommended doses, acetaminophen is a leading cause of acute liver failure due to intentional and unintentional overdoses.1 In the past decade, Health Canada has implemented several initiatives to improve the safe use of acetaminophen, including limiting the dose per unit (e.g., tablet or capsule) in prescription combination products and updating product labelling requirements.2,3 The impact of these labelling requirements was the subject of a recent review; the authors found that modifications to product labels did not reduce the rate of acetaminophen-related harm soon after implementation.4 Longer-term studies on the impact of product labelling and additional measures to optimize safe use of acetaminophen are needed.5 This bulletin shares information about initiatives underway in other countries and includes recommendations for improvements to product packaging (i.e., restricted pack sizes, child-resistant closures).
Discussion
There are approximately 4500 hospitalizations due to acetaminophen overdose each year, 16% (n=700) of which were reported as accidental or unintentional overdoses.1 Multiple factors contribute to acetaminophen-related harm.5 Factors can include the ease of access to potentially large amounts of the medication, whether it be a result of the availability of large quantities, a lack of child-resistant closures, or inadequate storage.5
Restricted Pack Sizes
The content of acetaminophen in many Canadian packages for adults far exceeds the typical single-ingestion doses involved in acute acetaminophen toxicity.6 The quantity of acetaminophen available in packages are limited in some other countries to reduce risk of harm (Table 1).
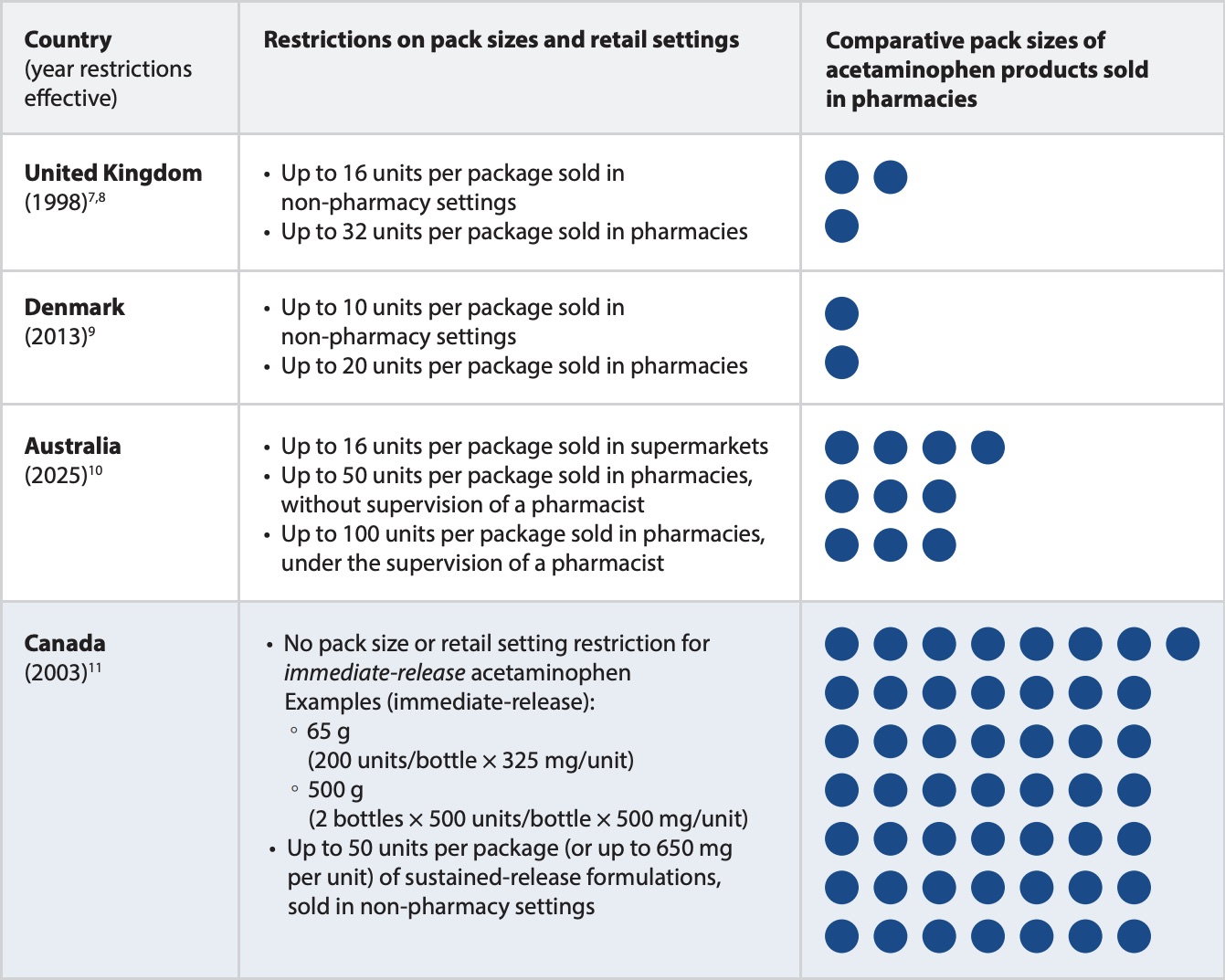
TABLE 1. Pack sizes of acetaminophen products for adults in select countries. (Note: units represent tablets or capsules; each circle represents 10 units).
Many European countries—including the United Kingdom, Denmark, and Sweden—have implemented pack size restrictions for over-the-counter sales of acetaminophen.12 Imposing restrictions on pack sizes has been shown to reduce the incidence of acetaminophen-related fatalities in the UK. Since 1998, the United Kingdom has enforced limits on acetaminophen packaging (Table 1).7,8 Recent data from England and Wales have shown a reduction in the number of deaths from acetaminophen toxicity, from 548 deaths in 1997 to 227 deaths in 2021.13
As of February 1, 2025, Australia will implement similar restrictions on package sizes (Table 1) with the aim of mitigating the harm caused by intentional acetaminophen overdoses. Limiting access to large amounts of acetaminophen may help to minimize potential harms associated with this medication.10
Child-Resistant Closures
Oral liquid acetaminophen products for infants, packaged with a child-resistant cap, may come with a separate dropper cap to help caregivers measure doses. Common caregiver practice is to replace the original cap with the dropper cap; however, not all of the dropper caps are child-resistant. The use of a product without a child-resistant dropper cap increases the risk of a child accessing the medication, which can lead to inadvertent poisoning.
Some packages of acetaminophen tablets marketed “for arthritis” may not have a child-resistant closure, to make it easier for patients with arthritis to open the container. Such products also make it easier for a child to open the container.

FIGURE 1. Example of an infant acetaminophen product with a child-resistant dropper cap. Not all infant products have this safety feature.
Safe Storage and Disposal
Safe storage and disposal14 of any unneeded medication, including acetaminophen, requires continuous patient awareness and efforts. Acetaminophen, like other medications, should be securely stored, out of sight, out of reach, and locked away from children, visitors, and pets. Parachute Canada has created a resource with suggestions for a proactive approach to safe storage: https://parachute.ca/wp-content/uploads/2020/04/ CheckForPoisons-Parent-and-Caregiver-Tips-toPrevent-Child-Poisoning-UA.pdf.
Recommendations
Health Care Practitioners
- Only carry and recommend infant’s and children’s acetaminophen products that have full child-resistant packaging (e.g., original cap AND dropper cap).
- Counsel patients/caregivers about the appropriate dose, recommended maximum daily dose,15 and directions for safe use of acetaminophen, including the following:
- acetaminophen content in other self-care products (e.g., cough and cold products)
- potential interactions (including with alcohol)1,16
- secure storage instructions (especially for products without child-resistant closures)
- disposal of expired or unneeded products by returning them to the pharmacy
- added risk when purchasing large quantities of acetaminophen
- If poisoning is suspected, contact the local poison centre through 1-844-POISON-X.17
- Consider patient-specific factors and therapeutic appropriateness before recommending analgesic medication. With increasing awareness of the potential for harm related to acetaminophen therapy, one might consider an alternative analgesic, including nonsteroidal anti-inflammatory drugs (NSAIDs) or opioids; however, these medications carry their own risks and may not be appropriate for use by certain patients.
- NSAIDs may increase the risk of gastrointestinal, renal, and cardiovascular adverse events.
- The adverse effects of opioids include sedation and constipation, and use of these drugs is associated with the risk of addiction and overdose.5
Health Canada, the National Association of Pharmacy Regulatory Authorities, and Manufacturers
- Review current evidence regarding the safest package size and format to reduce the risk for acetaminophen overdose.
- Ensure closures (e.g., original cap AND dropper caps) for all acetaminophen products intended for infants and children are child-resistant.
- Provide additional education and awareness about the safe use and potential harms of acetaminophen.

FIGURE 2. The key recommended strategies for safe use of acetaminophen are listed on the right alongside the Hierarchy of Effectiveness,18 which helps to illustrate relative impact with system improvement.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
