ALERT: Substitution Error with Tranexamic Acid during Spinal Anesthesia
ISMP Canada’s consumer reporting program, SafeMedicationUse.ca, received a report from a patient who was inadvertently given tranexamic acid into the spine as a result of a substitution error. They asked that their story be shared, “so that no one else would have to experience the same fate.”
INTRODUCTION
Inadvertent injection of tranexamic acid into the spine can lead to catastrophic patient harm; in an international review, approximately 50% of cases (n=21) resulted in death.1 Patients who survive such an error often experience long-term effects, such as neurological deficits.1 ISMP Canada’s consumer reporting program, SafeMedicationUse.ca, received a report from a patient who was inadvertently given tranexamic acid into the spine as a result of a substitution error. They asked that their story be shared, “so that no one else would have to experience the same fate.”
INCIDENT DESCRIPTION
The patient was scheduled for an orthopedic surgical procedure. In the operating room, tranexamic acid was inadvertently injected into the cerebrospinal fluid, instead of the intended local anesthetic, bupivacaine (Figure 1). The patient reported extreme pain and demonstrated unusual movements during the procedure, and experienced seizures while in the recovery room. Neither the anesthesiology team nor the surgical team recognized the medication error at the time. It was identified only when the same error occurred during a subsequent procedure later in the day.
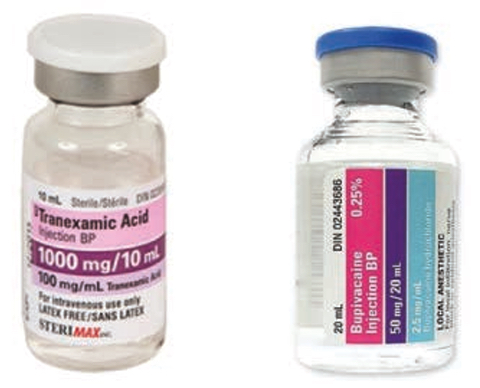
FIGURE 1. The tranexamic acid (at left) and bupivacaine (at right) products involved in the described incident.
Following this incident, the patient has been bed-bound and living with extreme chronic pain. They described how the error has permanently and significantly affected both their own quality of life and that of their family.
BACKGROUND
Tranexamic acid is an antifibrinolytic agent that is available in both oral and injectable formulations. The injectable formulation is intended only for intravenous (IV) administration2,3 and is typically indicated for the management of increased local fibrinolysis3 that can lead to severe bleeding. Tranexamic acid is neurotoxic if administered into the spine.4,5 As tranexamic acid is increasingly being used to reduce bleeding in obstetric, orthopedic surgical, and trauma patients, it is imperative that health care providers are aware of the potential for errors with harmful consequences.4,6
In 2020, the National Alert Network (NAN) in the United States issued an alert to highlight cases of dangerous wrong-route errors with tranexamic acid.7 Incidents reported in the alert resulted from mix-ups with local anesthetics such as bupivacaine and ropivacaine, which are available in vials (Figure 2). The international review of cases identified both the look-alike appearance with other medications and human factors as contributing to these types of errors.1 A Canadian case report of tranexamic acid administration into the epidural space has been recently shared.8
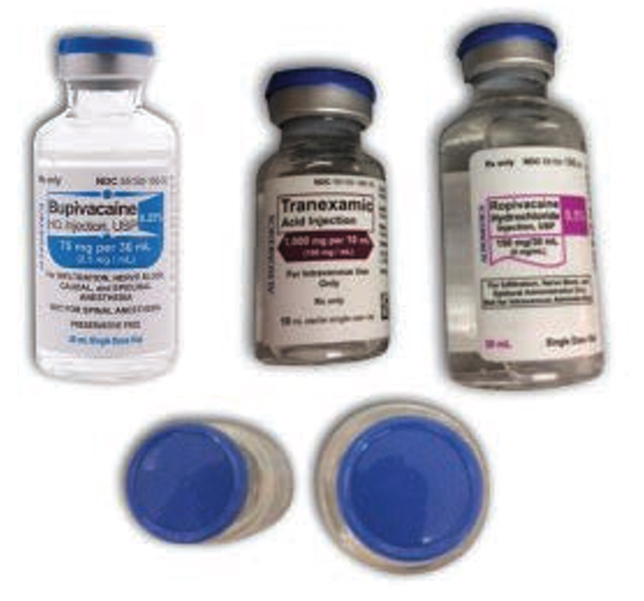
FIGURE 2. Tranexamic acid (middle product) and local anesthetics (bupivacaine on the left and ropivacaine on the right) involved in drug mix-ups in the US NAN Alert.
DISCUSSION
The hospital provided ISMP Canada with important information regarding the circumstances of the error and their response to reduce the risk of recurrence. At the time of the incident described above, both tranexamic acid and bupivacaine were stored in the same drawer of the drug cart. A double check of the vial label was performed when removed from the drawer, however, hospital analysis of the incident found that confirmation bias (i.e., the tendency to “see” information that confirms expectations)9 allowed tranexamic acid to be drawn up instead of bupivacaine. Investigation of an open bin of discarded vials (after the second patient was affected) confirmed that tranexamic acid had been mistakenly administered to the first patient.
Since then, the hospital has been in close communication with the patient and family to share several strategies that were implemented to prevent error recurrence. The hospital recognized the need for prompt action and additional layers of safety. A safety alert was immediately sent out hospital-wide and shared province-wide, and included the following strategies:
- Enhanced vigilance with dosing, preparation, and administration of medications via the spinal/ epidural route.
- A 2-person visual check of medication vials before spinal/epidural administration. The check includes verification of the drug name, concentration, expiry date, and a final check of the vial prior to administration.
- Application of a warning sticker on tranexamic acid vials to provide an additional visual cue.
Another strategy implemented by the hospital was to provide tranexamic acid for injection in a glass ampoule (Figure 3) to differentiate it from medications in vials. However, it is recognized that this change is not sufficient to prevent errors. A case report10 and the international review1 both described mix-ups between ampoules of tranexamic acid and local anesthetics.

FIGURE 3. An example of tranexamic acid supplied in an ampoule.
RECOMMENDATIONS
Inadvertent administration of IV medications by the neuraxial (spinal or epidural) route has been an area of concern for some time.4,11 Learning from this incident, along with previous recommendations to decrease the risk of this type of error, can improve the safe use of tranexamic acid:
Storage and Supply
- Store medications intended for IV administration (e.g., tranexamic acid) separately from those intended for neuraxial administration (e.g., local anesthetics).5,7,12-14
- Store medication vials in a way that makes the label visible to practitioners before selection. For example, where vials are stored in a drawer, they should be laid flat, with the label visible, instead of being placed upright (with only the vial cap visible).7,13
- Communicate any changes in medication supply with all hospital stakeholders and during unit-based safety huddles before the new product is distributed.
- Consult with key stakeholders when considering a change in medication suppliers to avoid the potential for look-alike medications in that practice setting.
- Consider additional signage in appropriate places (e.g., automated dispensing cabinets, storage cupboards) to communicate information about the new product labelling and packaging.15
Products and Equipment
- Consider additional safeguards typically used for high-alert medications, including warning labels.16
- Include a prominent warning on the tranexamic acid label that states, “FOR INTRAVENOUS USE ONLY”.2,3,7
- Consider attaching an auxiliary label to the vial cap stating, “Tranexamic Acid”.7
- Use equipment (e.g., syringes and infusion tubing) with neuraxial route-specific connectors (e.g., non-Luer lock) for spinal/epidural procedures.1,6-8,13,17
- Encourage medication suppliers to provide tranexamic acid for injection in diluted form, in a ready-to-use minibag for IV infusion,7,13 as is commercially available in other countries (Figure 4).

FIGURE 4. Example of a commercially available minibag of tranexamic acid outside of Canada.
Operating Room Processes
- Conduct an independent double check of any medication intended for neuraxial administration (with a second person or a barcode reader).1,7,13
- Reading aloud the label and incorporating mindful pauses into the verification process may be helpful.
- Promptly assess unusual or unexpected patient reactions or behaviour in the operating room to identify a potential adverse drug reaction or medication error, particularly when medications have been administered via the neuraxial route.
- Develop a method of disposal in the operating room that enables the review of discarded vials following a critical incident, while ensuring adherence to medical waste disposal policies.
Given the complexity of and variation in operating room set-up, processes, and practices, each hospital will need to develop its own multipronged approach to optimize the safe use of tranexamic acid.
ISMP Canada is currently collaborating with Health Canada and the Canadian Anesthesiologists’ Society to advance the safe use of tranexamic acid in Canada. To support this work, please continue to report near misses and incidents related to tranexamic acid injection, as well as share strategies that your hospital is considering or has implemented, to the Canadian Medication Incident Reporting and Prevention System (CMIRPS) (https://www.cmirps-scdpim.ca/?p=10&lang=en) or by email to info@ismpcanada.ca.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
