Introduction
A central fill service is a collaborative partnership between an “originating” pharmacy and a “central fill” pharmacy, with defined accountability and responsibility between them.1 Services provided by central fill pharmacies commonly include preparation of patient compliance packages, refill medications, and compounded prescriptions. After preparation by the central fill pharmacy, the completed prescription medications are sent to the originating pharmacy (where prescription orders are first received), where they are dispensed to patients (Figure 1).
With support from central fill services, originating pharmacies can save time, inventory costs, and staff resources, which can then be redirected to providing professional services to patients. However, without the appropriate processes and checks in place, the use of central fill services can add complexity to processes and can also blur accountability. This bulletin highlights key themes from a multi-incident analysis of incidents related to central fill services and shares error prevention strategies to address identified gaps.
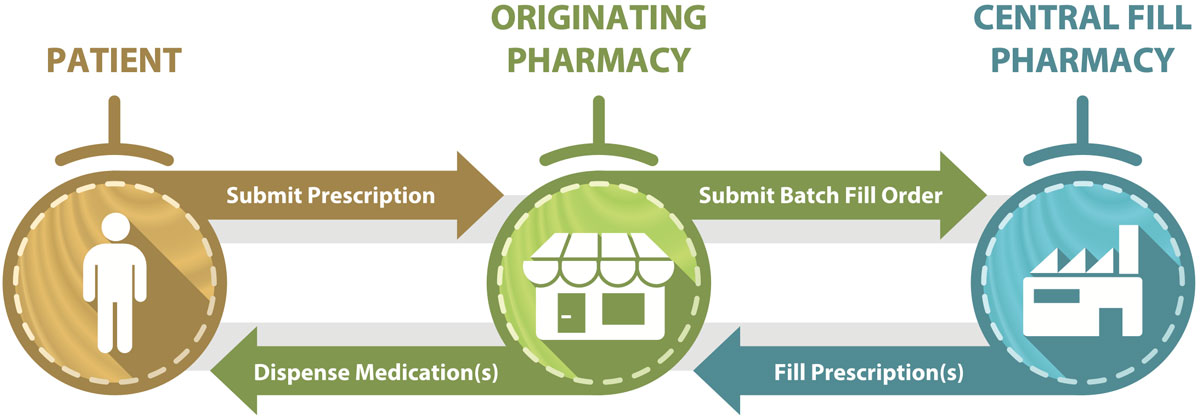
Figure 1. Flow of prescription information and medications if a central fill pharmacy is utilized.
Methodology
Medication incidents associated with central fill services were extracted from voluntary reports submitted to 3 ISMP Canada reporting databases (ISMP Canada’s Individual Practitioner Reporting, National Incident Data Repository for Community Pharmacies [NIDR], and Consumer Reporting) over the 2-year period from July 1, 2021, to June 30, 2023.
Search terms included but were not restricted to the following: “central*”, “batch”, and the brand names of automation machines commonly used in central fill pharmacies. Incidents unrelated to central fill services and duplicates of reported incidents were excluded.
A total of 713 incident reports were screened for inclusion, of which 185 met the inclusion criteria. The analysis was conducted according to the multi-incident analysis methodology outlined in the Canadian Incident Analysis Framework.2 The information provided by reporters in the description field yielded key learnings for the analysis.
Quantitative Analysis
Figure 2 shows the top 5 types of errors most frequently reported in the analyzed data set. The vast majority (98%) of incidents were concerns, near misses, or “no harm” events; mild harm to patients was reported in the remaining 2% of incidents.†
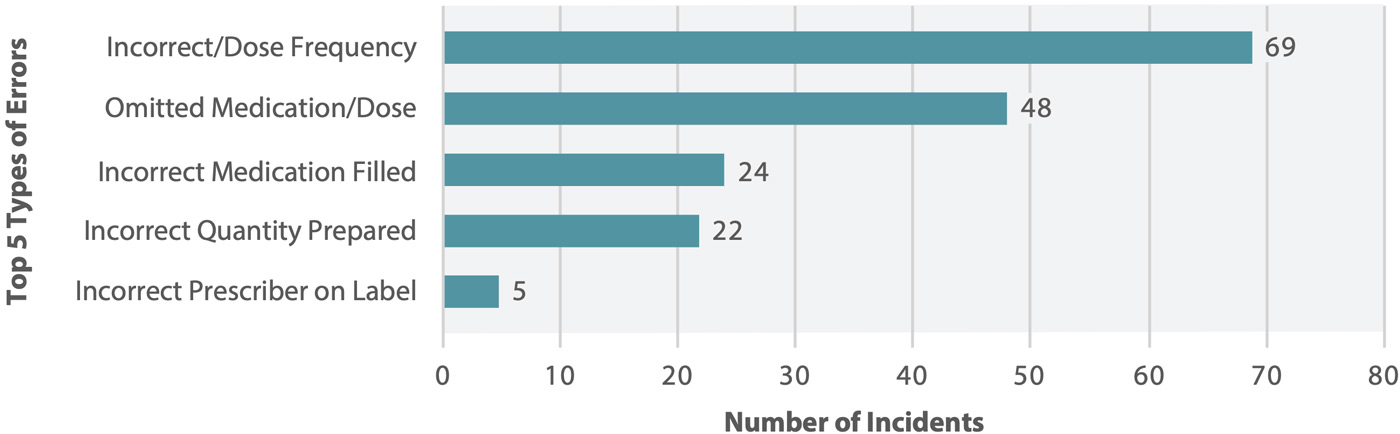
Figure 2. Top 5 types of errors reported in association with central fill processes
Qualitative Findings
The multi-incident analysis generated 3 themes and several subthemes. Figure 3 shows the themes and the relative frequency of incidents within each theme.

Figure 3. Themes and subthemes identified in the multi-incident analysis.
Theme: Automated Filling Process Workarounds in Central Fill Pharmacy
Automated filling processes use technology and machinery to package prescriptions. Such processes include automatic counting of pills, machine-labelling of prescription bottles, and technological processes to ensure accuracy. Automated filling has been shown to reduce the likelihood of filling errors, and some of these technologies can also improve error detection.3 However, for these benefits to be achieved, strong supporting processes are required.
In the current analysis, a “workaround” refers to a process that involves deviating from a standardized procedure, including bypassing or omitting a safeguard or automation step. Insufficient understanding of the importance of safeguards can lead to workarounds. Furthermore, high workload and automation complacency may lead to missed or inadequate quality checks.
Incident Example: During replenishment of the automation canister for rivaroxaban 15 mg tablets, a member of the central fill pharmacy team inadvertently filled it with rivaroxaban 20 mg tablets. The bar code on the medication bottle was not scanned before the canister was filled and inserted into the automated dispensing machine; high workload was reported as a contributing factor. The incorrect rivaroxaban strength was used to package orders, affecting patients’ care, until the error was discovered about 3 weeks later.
Theme: System Limitations
System limitations were reported as contributing factors in some errors in both central fill pharmacies and originating pharmacies. There are corporate formulary restrictions in central fill pharmacies, that may result in an inability to provide specified medications. Downtime issues can cause filling delays at central fill pharmacies. Downtime at the originating pharmacy was reported to affect the accuracy of the information submitted to the central fill pharmacy.
Theme: Order Submission Errors to Central Fill
Errors with Batch Fill Order Submission
A batch fill order refers to an order submitted to the central fill pharmacy by the originating pharmacy, with a request to prepare medications for one or more patients. A batch fill order is typically used for a specific dispensing cycle or schedule (e.g., some pharmacies send only their monthly compliance pack orders to a central fill pharmacy).
Factors contributing to reported errors associated with batch fill order submission included complex software that requires completion of multiple fields for batch fill order entries (leading to some elements being overlooked) and lack of structured processes to ensure completion of updates to patients’ medication profiles before submission of the batch fill order. Examples of reported errors included filling of previously discontinued prescriptions and omission of newly prescribed medications.
Incident Example: For 1 month, simvastatin was missing from a patient’s weekly compliance packs filled by the central fill pharmacy. The originating pharmacy had missed including simvastatin for batch fill at the time of order submission to central fill pharmacy. Staff at the originating pharmacy did not notice that simvastatin was missing before the packs were given to the patient.
Miscommunication of Post-submission Medication Changes
Post-submission medication changes occur when a primary health care provider makes changes to a patient’s medication therapy after the batch fill order has been sent. The lack of a standardized approach for dealing with post-submission medication changes, was reported to contribute to errors. For example, standardized communication and checking processes are important if changes are permitted.
Incident Example: A patient’s fluoxetine was changed to sertraline while their compliance packs were being processed by the central fill pharmacy. Although the central fill pharmacy was informed of the change, this notification did not occur soon enough to allow the change to be incorporated. The originating pharmacy was unaware that the change had not been processed by the central fill pharmacy. The incorrect medication was not identified during the originating pharmacy’s final clinical and technical check of the compliance pack and the patient received the wrong medication.
Conclusion
This multi-incident analysis has highlighted themes and incident examples associated with the use of central fill pharmacy services by community pharmacies. With 2 pharmacy entities involved in the dispensing process, expectations and accountabilities need to be clearly established and supported by standardized processes, including quality checks and communications. Community pharmacies are encouraged to review this bulletin; the shared learning informs quality improvement opportunities within their respective systems.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
