Confusion between Vaccine Expiry and Beyond-Use Dates Leads to Multi-Patient Incident
The introduction of vaccines to protect against COVID-19 was a turning point in the management of the pandemic. Knowledge about these products continued to evolve rapidly after they were made available, which resulted in frequent updates to stability and storage requirements and associated product labelling.
Introduction
The introduction of vaccines to protect against COVID-19 was a turning point in the management of the pandemic. Knowledge about these products continued to evolve rapidly after they were made available, which resulted in frequent updates to stability and storage requirements and associated product labelling.1-4 These changing requirements created a challenging work environment for health care providers who were administering the vaccines. This bulletin shares learning from an analysis of a multi-patient incident involving administration of COVID-19 vaccines that were past their beyond-use date (BUD).*
* The expiry date on a pharmaceutical product is the last date the manufacturer can guarantee the potency and safety of the product. When a product must be manipulated for use by health care providers, it is also given a beyond-use date (BUD), which reflects the latest date/time the manipulated product can be used.5
The COVID-19 vaccine product involved in this incident had 3 pertinent dates:
- a manufacturer-assigned expiry date that was applicable so long as the product remained in the frozen state;
- a BUD of 30 days once the product was placed in the refrigerator for thawing and storage; and
- a BUD of 6 hours once the vial was first punctured (i.e., when the diluent was added).
Incident Description
A health care organization in a small community was collaborating with the local public health unit to offer COVID-19 vaccination clinics. A nurse who was new to the organization was reviewing the vaccine supply and discovered that the available doses of vaccine were past their BUD. A subsequent review of vaccine administration records showed that more than 50 people had received the vaccine between 4 and 31 days past the BUD. A multilevel incident management team, including organizational and community leadership and public health experts, was immediately formed to determine the appropriate care response and to provide information to affected community members. Consultation by the incident management team with the vaccine manufacturer resulted in a recommendation for revaccination of about 40% of the individuals involved.
Background
Numerous media reports have highlighted errors related to the administration of expired COVID-19 vaccines worldwide.6-8 The Institute for Safe Medication Practices Canada (ISMP Canada) and its US counterpart, the Institute for Safe Medication Practices, have also received error reports. Analysis of these reports revealed the complexity of the process for managing COVID-19 vaccines (Figure 1) and identified potential points of vulnerability throughout. Recommendations to reduce the potential for error when administering these vaccines were shared in several bulletins and newsletters.9-11
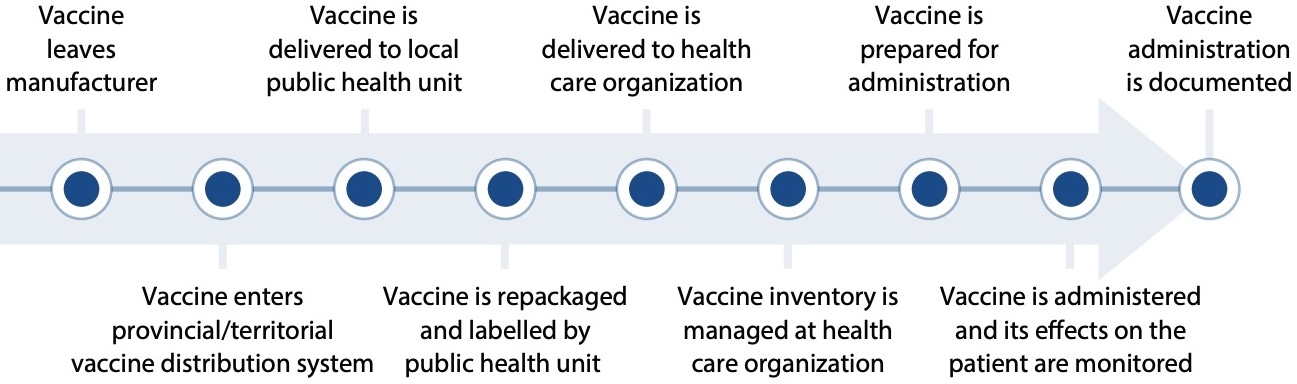
FIGURE 1. Example process of vaccine management before and after arrival at a health care organization.
Discussion
A comprehensive analysis of the incident described above was completed by a multistakeholder team that included representation from the community where the incident occurred. The analysis yielded 3 themes (Figure 2). The analysis findings and factors contributing to the incident for each theme are summarized in Themes 1-3.

FIGURE 2. Themes identified in the comprehensive analysis of the multi-patient incident.
Key mitigation strategies that worked well for the incident response are provided in Box 1 (see page 6).
The learning is generalizable to other settings and can assist with the safe management and administration of vaccines.
THEME: Vaccine Labelling and Packaging
Analysis Finding
The vaccine product required monitoring of 3 pertinent dates:
- Manufacturer’s expiry date for product in the frozen state
- BUD after placement in the refrigerator for thawing and storage
- BUD after first puncture for dilution
The BUD after placement of the product in the refrigerator for thawing and storage was not shown on any vaccine vials, which increased the likelihood that nurses would refer to the expiry date (intended to reflect the expiry if stored in the frozen state), which was shown on every vial.

Factors Contributing to the Incident
- The usual and expected practice for health care providers administering medication is to check the expiry date on the vial when preparing to administer a product.
- For this COVID-19 vaccine, the expiry date shown on the vial label (the date that can be predetermined by the manufacturer) was only applicable so long as the product remained in the frozen state.
- The vial label did not include instructions or space to document the BUD following placement of the product in the refrigerator for thawing and storage. The label only included instructions for determining the BUD after first puncture of the vial for product dilution, along with space for the health care provider to write that date and time.
- This vaccine was supplied in a bulk carton with large quantities, which necessitated product repackaging (and potentially thawing, as in this case) by the local public health unit before distribution to health care organizations requiring smaller quantities.
- Two labels were affixed to the lid of the plastic vaccine storage container sent from the public health unit to the health care organization (Figure 3):
- The label on the left provides non-clinically relevant information pertaining to return of the container.
- The label on the right includes the BUD of the repackaged vaccine after placement in the refrigerator for thawing and storage. The BUD information does not have prominence to distinguish it from other information on the label, nor was this information added to each individual vial. Additionally, the BUD was labelled as an expiry date. It is unclear from this label that “July 13, 2021” refers to the date when the vaccine was removed from the freezer and placed in the refrigerator.
- The lid of the plastic vaccine storage container was detachable and was not always kept with the box. As a result, the BUD was visible only when the lid was on the container
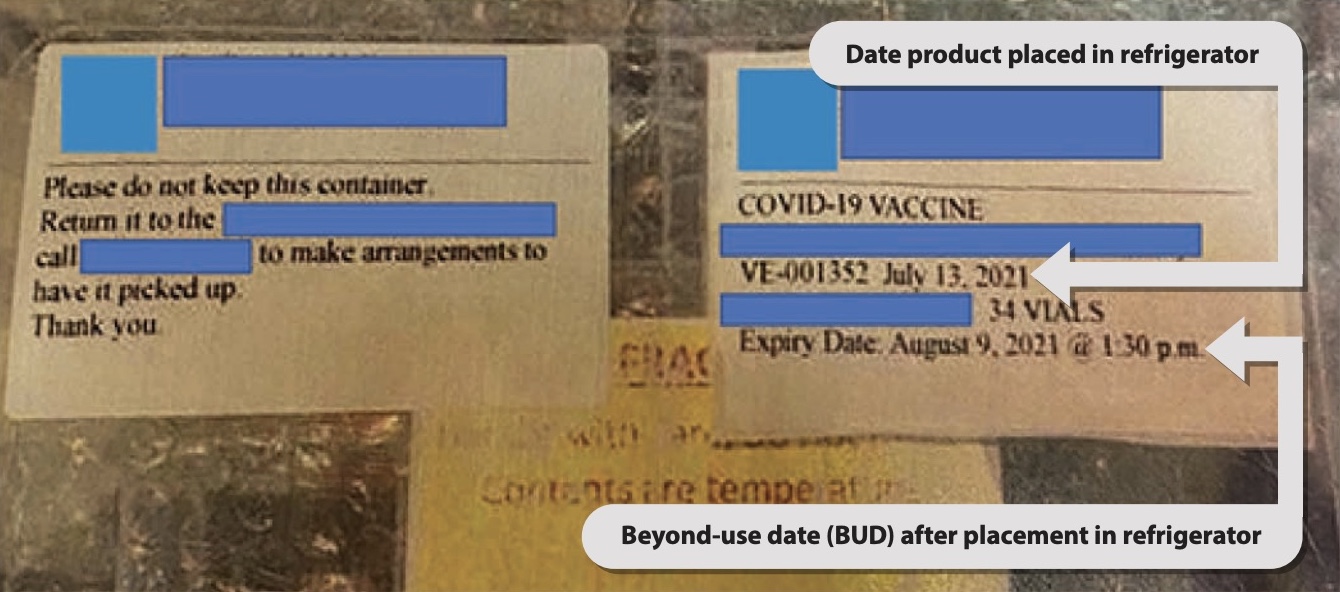
FIGURE 3. Labelling of vaccine container provided to the health care organization.
THEME: Vaccine Inventory Management
Analysis Finding
Responsibility for tracking and reviewing the BUDs of COVID-19 vaccines in the health care organization’s inventory was unclear and subject to competing priorities for nursing staff.

Factors Contributing to the Incident
- At the time of the incident, vaccine inventory management was one of many additional responsibilities that fell to nurses in the health care organization.
- Not all nurses had experience with vaccines, nor did they have specific expertise in managing medication inventories.
THEME: Availability of Vaccine Information
Analysis Finding
Vaccine information provided to front-line nurses was not easily accessible or understandable. As a result, the special handling requirements of this COVID-19 vaccine may have been unclear.

Factors Contributing to the Incident
- COVID-19 vaccines were new products with a more complex management process than other vaccines.
- There was little focus on expiry and BUD considerations during vaccine training. The training was focused on the important considerations of pharmacologic properties of COVID-19 vaccines, and appropriate vaccine selection and provision of information to patients.
- Information about COVID-19 vaccine products from several manufacturers was evolving rapidly, which made it dificult for organizations to keep front-line staff updated with changes to the handling requirements.
Analysis Finding
Determination of the appropriate clinical management for affected patients was time-consuming and difficult because only limited information was available from the manufacturer.

Factors Contributing to the Incident
- Initial clinical information received from the manufacturer following the incident was limited to content from the product monograph.
- Important, up-to-date information about stability of the involved vaccine was not readily accessible and required persistence of health personnel and use of personal contacts.
Recommendations
The following recommendations reflect the key learning from this analysis and highlight the need for action at multiple levels to prevent similar incidents in the future.
COVID-19 Vaccine Manufacturers
Labelling and Packaging
- If there is qualifying information for an expiry date (as was the case for this product, for which the expiry date was valid so long as the product remained in the frozen state), indicate such details alongside the expiry date.
- Provide space on the vial label or use an alternative label format (e.g., peelable, or separate label to be applied to the product) to support health care providers in documenting the BUD on individual vials, if it can differ from the expiry date (e.g., for different storage conditions).12
- Whenever possible, provide vaccines in prefilled unit-of-use syringes, stable in the refrigerator.
- In addition to current package sizes, provide vaccine products in packaging that will support lower volume use (e.g., cartons of 10–12 vials) to avoid the need for repackaging for further distribution.
Medical Information
- Describe potential errors and ways to avoid them in educational and communication resources to support safer use of vaccine products.
- Establish or enhance a process to escalate questions received from health care providers to ensure that timely advice can be provided when a question arises that cannot be adequately answered from the product monograph.
- For questions about new products received from users, develop FAQ responses that can be readily shared if the same questions arise in the future.
Public Health Units and Other Agencies/ Organizations Responsible for Vaccine Distribution
- Design labels with the end-user in mind. Carefully consider the information that will be needed and give prominence to critical information.
- Consider font size and format (e.g., colour, type colour, and use of bold type) for labels. If other, noncritical information is deemed necessary, position it so that it is clearly secondary to the critical information.
- Ensure that critical information remains with the product at the point of use.
Health Care Organizations Administering COVID-19 Vaccines
- Consider the following strategies to reduce the cognitive workload for staff involved in managing COVID-19 vaccines:
- Develop concise practice aids/work instructions to support nurses and other health care providers in vaccine administration (e.g., checklists, single-page information sheets).
- Develop a checklist for vaccine inventory management that includes, at a minimum, key information that health care providers must check, such as BUD(s), and instructions for managing vaccine doses that are left over after a planned vaccine clinic.
- Ensure training and supporting materials clearly discuss the differences between expiry dates and BUDs, as well as the established protocols for inventory management and documentation (e.g., BUD on vial label).
- Implement a record system for tracking vaccine inventory. Ideally, provide a prepopulated vaccine log sheet that includes the expiry date and BUDs as a visual reminder of key elements to check.
- Implement an independent double-check process that includes a check of the BUDs during the vaccine selection, preparation, and administration steps.
Readers are also referred to resources available from the Public Health Agency of Canada, Health Canada, and the United States Pharmacopeia to support practitioners in the safe storage and handling of COVID-19 vaccines.13,14,15 The Public Health Agency of Canada previously published a document describing immunization competencies.16
BOX 1. Key Mitigation Strategies that Worked Well for the Incident Response
- Nurture a culture of safety that empowers staff and community members to identify safety gaps and to advocate for the community.
- Establish an incident management team when a patient safety incident occurs. Include an incident lead, a communications lead, and representatives from local community leadership, where applicable (e.g., incidents involving multiple patients).
Conclusion
The findings from this analysis emphasize the information needs of health care providers at all steps in the vaccine management process. The clarity and prominence of critical information on vial and supplementary labels, and the ease with which important information can be interpreted, are crucial to the safe use of a product.17 Designing a label and package for safe use requires consideration of the end-users, as well as the environments and processes in which the products will be used. Development of related policies, procedures, and training materials also requires consideration of similar principles to best support health care providers involved in vaccine management, including inventory controls and BUD monitoring, and vaccine administration.
This incident analysis also highlights the importance of engaging the community and learning the perspectives of patients, families, and caregivers. All stakeholders involved in the production, distribution, preparation, and administration of new vaccines are encouraged to review the findings from this analysis. This can identify areas of vulnerability in vaccine-use processes in their own practice settings and system strengths that should be sustained.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
