INTRODUCTION
Administration of medication by the intravenous (IV) route (commonly referred to as IV medication administration) is ubiquitous in hospital practice. This route of administration is often used for several high-alert medications that bear a heightened risk of causing significant patient harm when used in error.1 The need for improvements to the medication-use process, predicated on an awareness of the risk of IV medication-related harm, is an important step toward system-level changes. A multi-incident analysis was conducted to inform the future direction of medication safety efforts specifically targeting administration by the IV route.

METHODOLOGY
Medication incidents associated with IV medication administration were extracted from reports submitted to 3 ISMP Canada reporting databases (Individual Practitioner Reporting, Consumer Reporting, and Community Pharmacy Incident Reporting) and the Canadian Institute for Health Information’s National System for Incident Reporting (NSIR) database† over the 3-year period from October 2015 to September 2018.
Key terms used to search the databases included “drip”, “IV”, “intravenous”, and “infus*”. Incidents were excluded if they described only the use of unaltered commercial IV fluids (i.e., with or without electrolytes, but without any additional additives), blood products, or total parenteral nutrition/feeds, or if they involved the IV administration of a medication intended solely as a “rescue” agent (in response to a previous medication error).
QUANTITATIVE FINDINGS
A total of 2210 incidents were identified and screened for inclusion. Of these, 1583 incidents were included in the quantitative analysis.¥ The key quantitative findings are provided in the following tables and figures, including the top 10 medications, the top 3 medication-use stages, and the top 5 types of errors most frequently reported in all incidents, as well the top 5 medications reported to be involved in harmful incidents.
TABLE 1. Top 10 medications most frequently reported to be involved in IV medication incidents
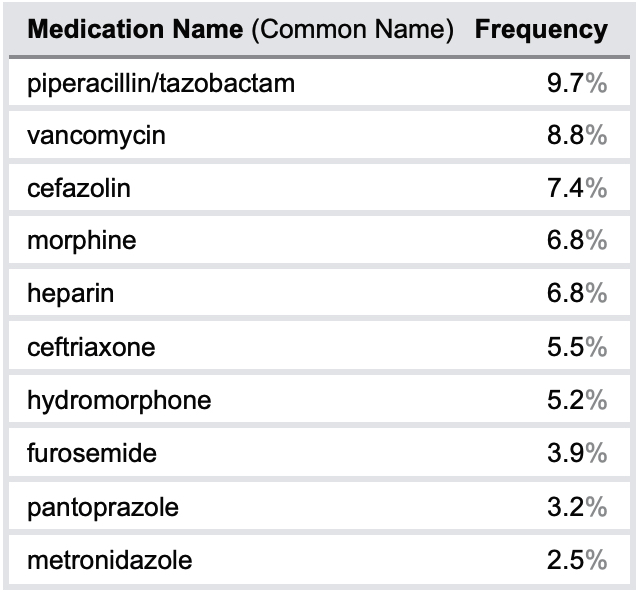

FIGURE 1. Top 5 medications most frequently reported to be involved in IV medication incidents causing harm

FIGURE 2. Top 3 medication-use stages most frequently reported to be involved in IV medication incidents

FIGURE 3. Top 5 types of errors most frequently reported to be involved in IV medication incidents
QUALITATIVE ANALYSIS
After exclusion of reports that lacked sufficient narrative detail to ascertain the circumstances of the incident, 1498 incidents were included in the qualitative analysis, conducted according to the methodology outlined in the Canadian Incident Analysis Framework.2 Qualitative analysis of the incident report narratives revealed 3 main themes, each with multiple subthemes (see Figure 4).
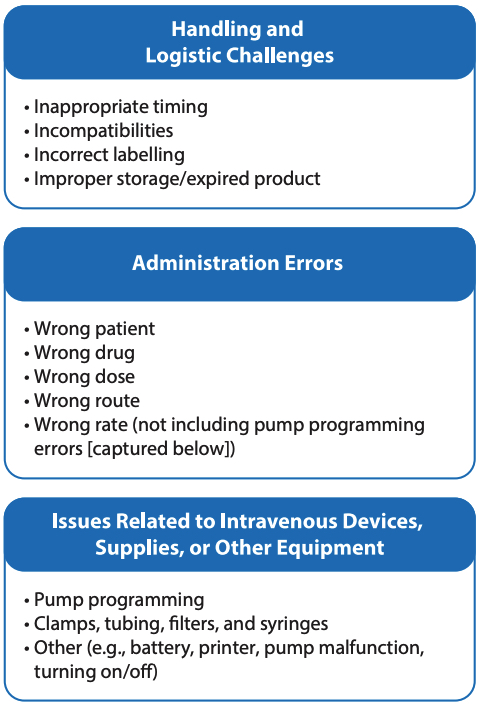
FIGURE 4. Main themes and subthemes
THEME: Handling and Logistic Challenges
This theme, identified in almost half of the incidents analyzed, encompassed all reports in which incompatibilities, scheduling, timing, storage, preparation, or labelling of the IV medication (or any combination thereof) was indicated as a contributing factor. For example, unclear or ambiguous documentation in the medication administration record (MAR) led to scheduling and timing errors in some cases. Confusing pharmacy-applied labels3 contributed to some incidents, such as programming errors (e.g., calculation instructions for a pharmacy technician on the label were misread as programming information for the nurse), as well as errors in storage conditions for the medication (e.g., medication not stored at the correct temperature). Errors also occurred when the drug concentration and infusion rate appearing on the MAR and on the infusion bag label did not align with the pump programming units and sequence.4 Delays or miscommunication related to transport or portering of medication were also contributing factors identified within this theme.
Hospitals are encouraged to facilitate close collaboration between the nursing and pharmacy teams to optimize the logistics and handling of IV medications.
THEME: Administration Errors
The occurrence of some type of administration error was the second most common theme, with approximately one-third of the incidents falling into this category. The administration errors identified in this analysis involved the wrong patient, the wrong drug, the wrong dose, the wrong route, or the wrong rate (not including pump programming errors). The lack of an independent double check was a factor in many of these incidents. Over-reliance on smart pump and bar-code technology, without an independent double check before IV medication administration (to verify and document the patient’s name, the drug and its concentration, the prescribed infusion rate, the line attachments and labels), was a key contributing factor in many of these incidents.
Hospitals are encouraged to continue to focus quality improvement initiatives on accurate IV medication administration.
THEME: Issues Related to IV Devices, Supplies, or Other Equipment
In this analysis, about 1 in every 6 reports identified contributing factors related to the pump used for IV medication administration (e.g., incorrect programming, pump malfunction) or with an associated accessory (e.g., missing filter, correct tubing not available). The reports identified that inexperienced or relief staff may not be familiar with the accessories, the sequence of programming steps, or the metrics that are preprogrammed into the pump. A study noted that with many different IV pumps available on the market, the user interface and unfamiliarity with the pump play a role in programming errors.5 Key system-level concerns were reported to be incomplete drug and rate libraries in the pump software, improper labelling of the IV line, transposition of IV lines after temporary disconnection, pump failure due to inadequate maintenance and/or battery charge, and printer-related issues, such as low ink levels resulting in illegible reports.
Hospitals should ensure that staff are trained on the use of infusion pumps on a regular basis to maintain up-to-date knowledge and skills in pump set-up and programming.6 Additionally, hospitals and purchasing groups are encouraged to collaborate with product manufacturers for continuous quality improvement efforts, including standardization of pump accessories and drug library information fields.
CONCLUSION
Medications are commonly administered by the IV route in many health care settings. This multi-incident analysis has characterized the types of medications and incidents that were most frequently reported with IV administration and raises awareness of opportunities to improve patient safety. Notably, among the medications most often reported to be involved in harmful incidents were 3 high-alert medications, including 2 opioids. Strengthening medication handling and logistics, incorporating appropriate checks prior to medication administration, and mitigating potential device-related issues are all needed to improve IV medication safety.
