Medication Incident Data in Canada: A Strategy for More Effective Sharing and Learning
Knowledge gained from a local analysis of medication incidents could benefit other healthcare providers and organizations at the provincial, national, and international levels. A cohesive, formal information-sharing strategy would facilitate a better understanding of reported medication incidents and would support the development of robust strategies for preventing patient harm, as well as creating a dynamic and supportive mechanism for shared learning.
INTRODUCTION
Most healthcare organizations providing patient care in Canada independently collect and analyze medication incident data; and many of these organizations develop and implement recommendations arising from their investigations. The reporting and analysis of medication incidents, is one way stakeholders can become aware of medication safety risks, as well as the factors that may contribute to harmful incidents.1 The valuable knowledge gained from a local analysis could benefit other healthcare providers and organizations at the provincial, national, and international levels.
One mechanism to disseminate and increase awareness of internationally reported information is the Global Patient Safety Alerts program, run by the Canadian Patient Safety Institute (CPSI). This makes available a collection of indexed patient safety incidents (in the form of alerts and advisories), recognized by the World Health Organization (WHO) and its member countries.2
A Medication Safety Action Plan3 was a key product of the Medication Safety Summit in 2014,4 with the express goal to, “develop a mechanism by which medication information and learning from different data sources can be shared effectively” in Canada.3 A cohesive, formal information-sharing strategy would facilitate a better understanding of reported medication incidents and would support the development of robust strategies for preventing patient harm, as well as creating a dynamic and supportive mechanism for shared learning.3 The undertaking was divided into 3 phases (Figure 1).
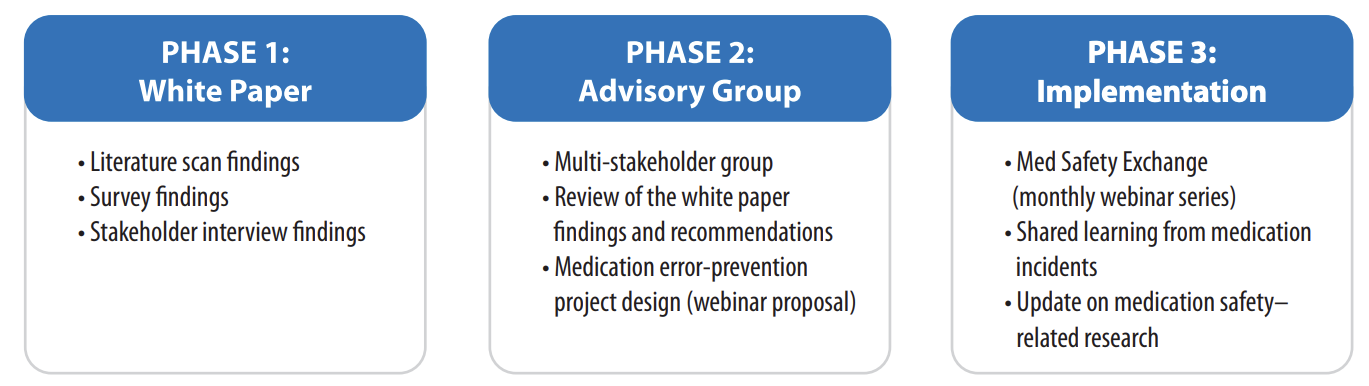
Figure 1: Overview of the key tasks and outcomes of the 3 phases of the project
PHASE 1: WHITE PAPER ON MEDICATION INCIDENT REPORTING IN CANADA
The white paper on medication incident reporting in Canada, released in late 2016, was jointly authored by the Canadian Institute for Health Information (CIHI) and ISMP Canada, with funding from CPSI, and incorporated multidisciplinary input from across Canada.5 The report describes findings and recommendations from the environmental scan of Canadian literature, a survey of practices at diverse Canadian health service organizations, and a series of stakeholder interviews.
Key recommendations from this white paper are to improve both the quantity and quality of medication incident reporting and to improve the linkage of reporting systems.5 The recommendations focus on promoting development of a network of medication incident repositories and expanding awareness of available reporting systems and portals among all types of reporters. Collection and integration of reports would create a larger pool of data within which error and prevalence patterns could more easily be recognized. Implementing corrective measures as a result of reported medication incidents would then serve to further incentivize reporters to use and support the reporting process.
PHASE 2: MEDICATION SAFTEY ADVISORY GROUP
A national group of representatives from multiple stakeholder organizations (Box 1) convened to develop a program to improve sharing of and learning from medication incident data across Canada. The group was tasked with applying the findings, recommendations, and key messages from the white paper toward the design, implementation, and evaluation of the proposed strategy.
The group recognized that several different data standards and taxonomies are currently in use by the many existing reporting systems. Therefore, it was proposed that sharing the incident data analyses, rather than the raw data underlying those analyses, would be easier, more educational, and more useful for the multiple participating organizations.

Box 1: Membership of the Medication Safety Advisory Group
The group conceived a monthly webinar series focused on medication safety, as described in the following section. The initial format outlined for the monthly webinar includes quality improvement evaluation measures, which will contribute to the continued development of the webinar program.
PHASE 3: MED SAFETY EXCHANGE WEBINARS
The goal of the Med Safety Exchange webinar series is to facilitate shared learning from medication incident data analyses. It is intended to be a national, practitioner-driven online platform, with interactive components. Each 1-hour webinar will contain several short presentations from multiple healthcare organizations, followed by a review of recent regulatory warnings and alerts, as well as a discussion of the recent academic literature in medication safety. Opportunities to submit questions and comments will be provided. In this way, those in attendance can identify similar vulnerabilities and/or safety opportunities in their own systems, or contribute their own strategies for dealing with identified medication safety issues. The recorded webinars will be available on the webpage within one week of the live presentation.

The Med Safety Exchange aims to increase participants’ awareness of key findings and recommendations from incident data, as well as current medication safety-related research and Health Canada warnings. Presentations from a wide range of organizations will offer a variety of approaches and perspectives. Additionally, participation in the Med Safety Exchange will validate the benefits of error reporting in optimizing medication safety for patients, thereby promoting and reinforcing the value of the reporting systems themselves.
CONCLUSION
The overarching goal proposed by the Medication Safety Summit was to develop a new platform for effectively sharing and learning from medication incidents reported across Canada. The steps in this process, described above, have led to the upcoming complimentary Med Safety Exchange webinars. Healthcare providers and organizations from all types of care settings are encouraged to participate in the monthly series beginning September 13, 2017.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
