Medications Most Frequently Reported in Harm Incidents over the Past 5 Years (2015-2020)
Key activities within the CMIRPS program include the capture and analysis of medication incidents (including harmful events, no-harm events, near misses, and circumstances that could lead to harmful incidents), as well as dissemination of learning to inform and support actions to improve the safety of medications for Canadians. This bulletin shares findings from analyses of incidents reported to the CMIRPS reporting components over a recent 5-year period.
INTRODUCTION
The Institute for Safe Medication Practices Canada (ISMP Canada) has a vision of Zero Preventable Harm from Medications. To help attain this goal, the organization is one of five national partners in the Canadian Medication Incident Reporting and Prevention System (CMIRPS – see Figure 1).1
Key activities within the CMIRPS program include the capture and analysis of medication incidents (including harmful events, no-harm events, near misses, and circumstances that could lead to harmful incidents), as well as dissemination of learning to inform and support actions to improve the safety of medications for Canadians. This bulletin shares findings from analyses of incidents reported to the CMIRPS reporting components over a recent 5-year period.
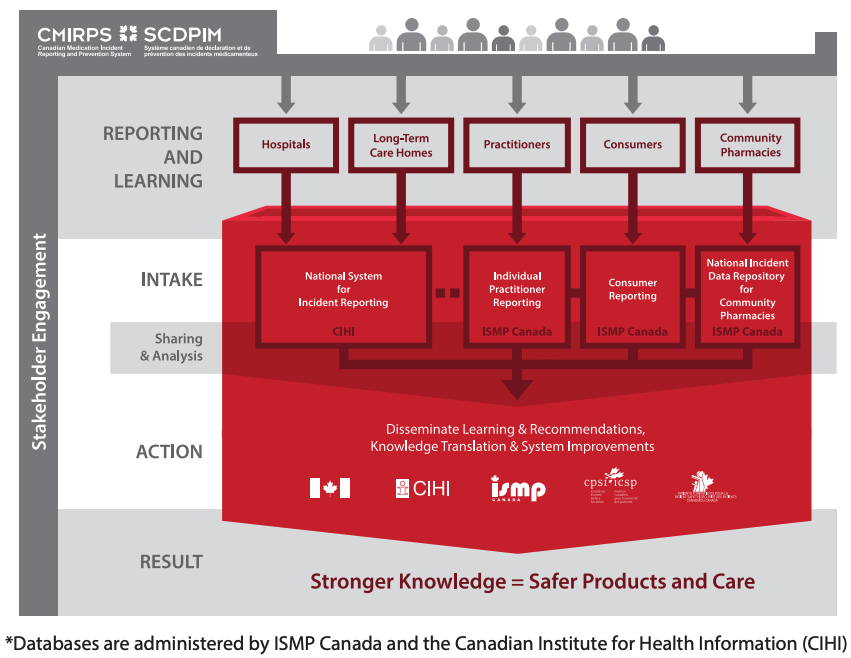
FIGURE 1. An Overview of the Canadian Medication Incident Reporting and Prevention System*
METHODOLOGY
Reports of medication incidents resulting in harm (“harm incidents”) were retrieved from the 4 CMIRPS databases:1 ISMP Canada’s Individual Practitioner Reporting, Consumer Reporting, and National Incident Data Repository for Community Pharmacies and CIHI’s National System for Incident Reporting2 (NSIR). Data were extracted for the 5-year period from January 27, 2015 to January 26, 2020.
The incident reports were reviewed to exclude duplicates and those in which the medication could not be identified. The remaining reports were then sorted by medication, such that all reports for a given generic medication, including various brand names, were grouped together. The most frequently cited medications involved in harm incidents were identified and then categorized by health care setting (hospital, long-term care, community pharmacy, and home and community care) and degree of harm (i.e., mild, moderate, severe, death).
QUANTITATIVE FINDINGS
A total of 7531 harm incidents were included in the analysis: 5234 and 2297 from NSIR and ISMP Canada databases, respectively. A key finding is that nearly 86% of all harm incidents were reported as mild in severity, as seen in Figure 2. Reports from hospitals form the majority of the incidents, followed by those from community pharmacy (Figure 3).
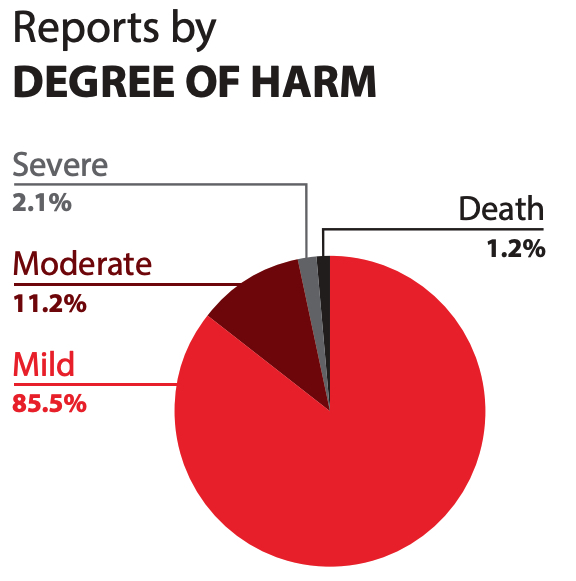
FIGURE 2. Degree of harm reported in harm incidents over a 5-year period from Jan 27, 2015 to Jan 26, 2020
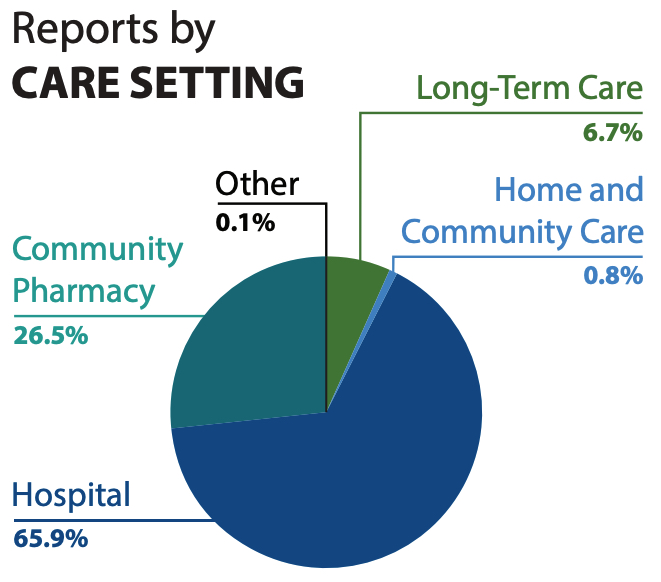
FIGURE 3. Reported care setting where harm incidents occurred over a 5-year period from Jan 27, 2015 to Jan 26, 2020
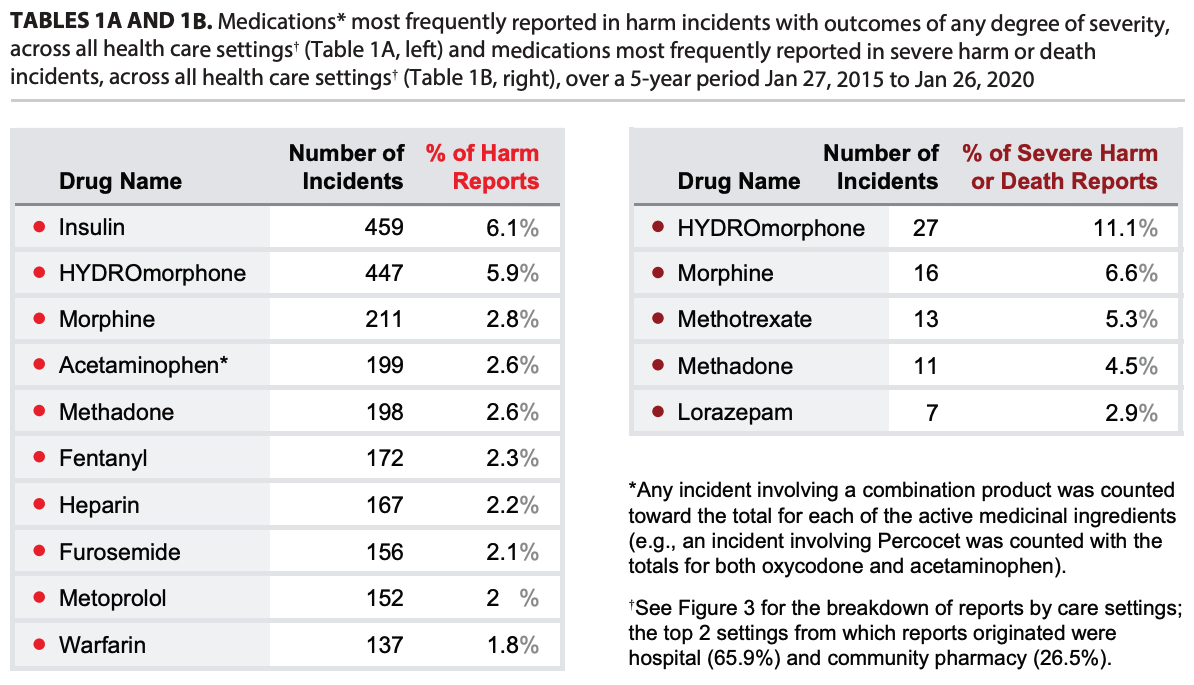
Figure 4 highlights the top 3 medications cited in reports of harm incidents in each health care setting. Two medications appear in the top 3 in multiple settings—hydromorphone in all except community pharmacy and insulin in the 2 institutional care settings. Notably, each of these 2 medications was cited twice as often as any other medication in harm incidents from all health care settings combined (Table 1A).
The data from all health care settings for the 5-year period were compiled to identify the medications most frequently cited in reports of harm incidents with outcomes of any degree of severity (Table 1A) and those reported to have resulted in severe harm or death (Table 1B).
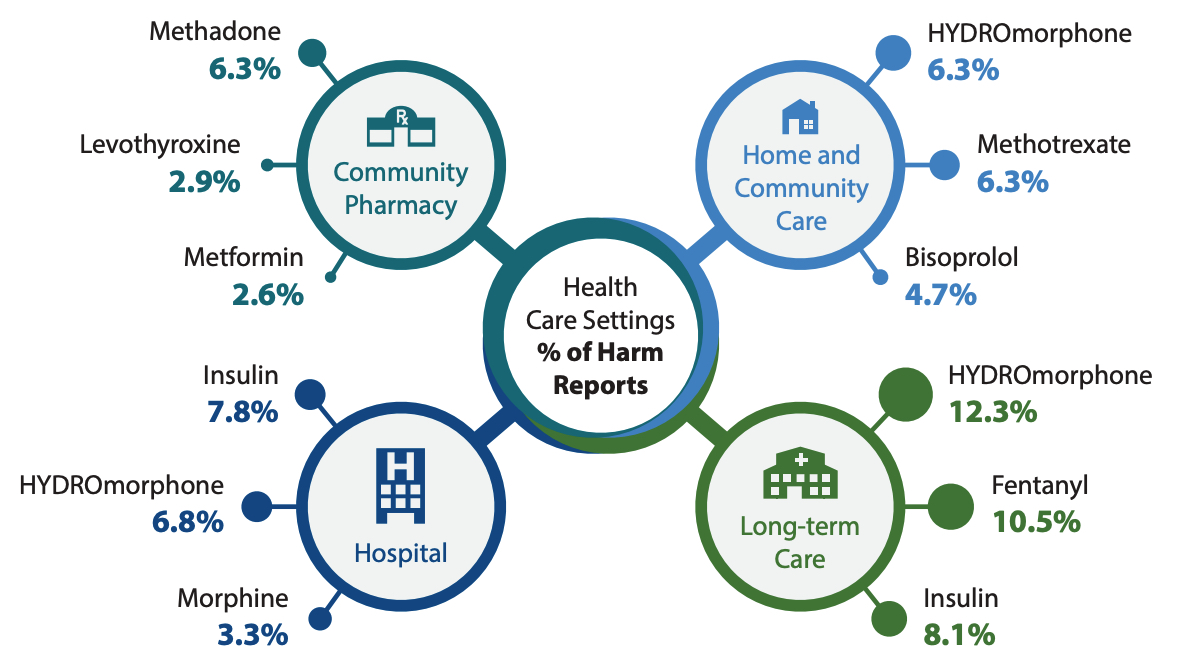
FIGURE 4. Medications most frequently cited in reports of harm incidents, by health care setting, over a 5-year period Jan 27, 2015 to Jan 26, 2020
.
DISCUSSION
All 5 of the medications most frequently reported in severe harm or death incidents across all care settings over the past 5 years are defined as high-alert medications.3,4 For example, methotrexate for non-oncologic indications is considered to be a high-alert medication in the community and ambulatory setting.5 Contributing factors associated with severe harm related to methotrexate were dosage errors where doses prescribed for weekly administration were taken daily, unrecognized drug interactions, and inappropriately high doses prescribed for patients who were experiencing renal failure. Patients, families, and health care providers may not appreciate the increased risks associated with this medication, nor be aware of the best practices recommended to prevent associated harm and death.6
Although awareness of high-alert medications and the need for related safeguards has increased over time, findings from the analyses in this bulletin serve as an opportune reminder that these and other medications continue to cause patient harm. There are known effective strategies for reducing the risk for error and harm for high-alert agents.7 Safety strategies and risk reduction measures, including optimization of technology, process improvement, patient/caregiver/health care provider education, and enhanced patient monitoring systems must be implemented.8
The analyses highlight findings that merit further investigation:
- Hydromorphone was among the top 3 drugs reported in harm incidents in 3 out of the 4 health care settings. Insulin was also frequently reported in harm incidents across multiple health care settings.
- In the community pharmacy setting, methadone was associated with the highest number of harm incidents. Insufficient patient identification processes, the need for individualized dosing regimens, and complex preparation steps were all contributing factors identified.
- Two medications reported as causing harm (acetaminophen and furosemide in Table 1A) are not identified high-alert drugs. Some reports that included acetaminophen involved a combination product containing this drug and an opioid (e.g., codeine, oxycodone).
- The appearance of levothyroxine in the list of incidents from community pharmacies may be influenced by the frequency of prescribing and dispensing this drug. Nonetheless, its presence in harm reports merits investigation into the root causes and contributing factors leading to patient harm.
LIMITATIONS
The reports submitted likely represent only a subset of the actual medication errors that are occurring. It is not possible to infer or project the probability of incidents on the basis of voluntary reporting systems.
CONCLUSION
Consumers, practitioners, health care organizations, and regulators can use the findings presented in this bulletin to inform the development of strategies for improving medication safety. One such strategy is the use of Medication Safety Self-Assessment tools focusing on “never events”; a resource is currently available for long-term care and another for hospitals and ambulatory care centres, while a third is in development for community pharmacy.9 These self-assessments support identification of practice and system vulnerabilities in each of the care settings and suggest safety strategies to prevent and mitigate harm.
The value of reporting medication errors to CMIRPS for national analyses and shared learning is increasingly being recognized across health care settings. The findings from these analyses provide important insights into medications most frequently reported in association with preventable harm and death in Canada.

Medication Safety Self-Assessment: Focus on "Never Events" in Hospitals and Ambulatory Care Centers
Sponsors
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
To receive ISMP Canada Safety Bulletins and Newsletters visit: https://ismpcanada.ca/safety-bulletins/#footer
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2024 Institute for Safe Medication Practices Canada.
