Penicillin Formulation Mix-Ups Lead to Potential Undertreatment of Syphilis
This bulletin highlights a mix-up between 2 penicillin formulations, the proactive response of a hospital to identify other potentially affected patients, and recommended changes to the medication-use system to prevent similar incidents from occurring.
Sidebars:
- Check the Syringe! Preventing Methotrexate Dose Errors
- Canadian High-Alert Medication List: We Want to Hear from You!
INTRODUCTION
Syphilis is a sexually transmitted infection that is on the rise in Canada.1 Syphilis is caused by the bacterium Treponema pallidum. First-line treatment for primary, secondary, and early latent syphilis is penicillin G benzathine given by intramuscular (IM) injections. Untreated or undertreated syphilis can be transmitted to others and can also advance to severe neurologic, cardiac, and musculoskeletal sequelae and even death.1 This bulletin highlights a mix-up between 2 penicillin formulations, the proactive response of a hospital to identify other potentially affected patients, and recommended changes to the medication-use system to prevent similar incidents from occurring.
INCIDENT EXAMPLE
Penicillin G benzathine 2.4 million units IM was ordered for a patient who presented to an emergency department (ED) and was diagnosed with syphilis. Penicillin G sodium 2.4 million units was inadvertently retrieved and administered IM. A nurse identified the error after finding penicillin G benzathine prefilled syringes in the ED refrigerator. The hospital investigated this incident and subsequently identified many similar incidents involving inadvertent administration of penicillin G sodium in the treatment of suspected or confirmed syphilis.
BACKGROUND
Syphilis is an important public health concern, as the rate of this infection increased by 153% from 2014 to 2018.1 Penicillin G benzathine is the preferred antibiotic treatment for syphilis because its long-acting properties make a single dose suitable for treatment.1 The current Canadian guideline recommends penicillin G benzathine 2.4 million units given IM as a single dose through 2 injections at different sites for infectious syphilis of selected subtypes (primary, secondary, and early latent stages).2 Syphilis of longer duration, such as latent or late latent syphilis, requires up to 3 weekly IM doses of penicillin G benzathine 2.4 million units. Penicillin G sodium IV may also be required (on its own or in addition to the benzathine formulation) for treating syphilis that involves the brain, ear, or eye.3
In Canada, penicillin G is available in multiple salt formulations, including benzathine and sodium. Both products contain penicillin, but they are not interchangeable. Table 1 outlines selected differences between the 2 salts.
Mix-ups between the sodium and benzathine salts of this drug can lead to patient harm:
- Undertreatment of syphilis when penicillin G sodium is administered instead of penicillin G benzathine, as described in the incident above.4
- Cardiopulmonary arrest and death when penicillin G benzathine is confused with penicillin G sodium and administered IV.4,5
These potential outcomes underscore the need to understand the differences between the salts.

TABLE 1: Selected differences between the penicillin G salts, benzathine and sodium, in relation to the treatment of syphilis.4,6
DISCUSSION
Upon discovery of the error, the hospital recognized that incidents of this nature were likely to have occurred before. Medical records were searched, additional incidents were identified, and a multi-incident analysis was performed.
The following contributing factors were identified:
- lack of awareness and/or understanding of the existence of multiple penicillin G salts, with different pharmacological properties, indications for use, and storage requirements
- incompleteness of orders written on paper prescriptions (Table 2)
- lack of pharmacist review of paper-based orders for non-admitted patients treated in the ED
- familiarity with the availability of penicillin G sodium in the automated dispensing cabinet.
The hospital recognized the need for prompt action and additional layers of safety. Incidents were disclosed to patients and/or clinicians, as appropriate, with support from Patient Relations and provincial public health communicable disease representatives.
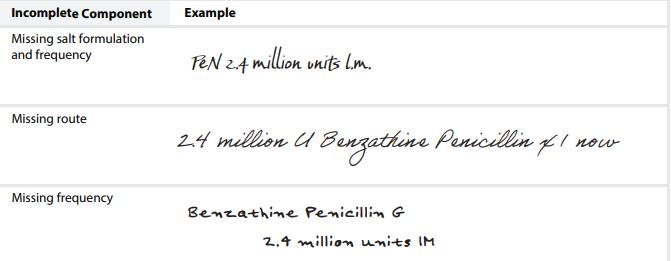
TABLE 2. Examples of incomplete written orders contributing to errors (replicated to avoid identication).
RECOMMENDATIONS
A multidisciplinary team, including prescribers, nurses, pharmacists, health informatics specialists, community care providers, and hospital administrators, analyzed the incidents. Several system-based recommendations to improve the medication-use process are shared below, including those developed by the hospital team.
Prescribers
- Include the salt formulation, dose, route, frequency, and duration for any penicillin prescription.
- When prescribing penicillin G, specify the indication in the order
Pharmacy Team
- Verify orders for penicillin G, when possible, to ensure the correct salt formulation for the indication is prescribed, dispensed, and administered.
- Apply auxiliary labels to penicillin G benzathine products that specify, “For Intramuscular Use Only”.
Nursing Team
- If penicillin G is ordered without specification of the salt formulation and the indication is unclear, contact the prescriber to verify the intended salt.
Health Informatics Personnel
- In electronic prescribing systems and/or pharmacy information systems, ensure that orders for penicillin G sodium default to the IV route and that orders for penicillin G benzathine default to the IM route.
- Add alerts for penicillin G benzathine in electronic systems indicating, “For Intramuscular Use Only” and “In Refrigerator”; these alerts should be visible on paper and electronic medication administration records
- Consider building decision supports into the electronic prescribing systems, pharmacy information systems, nursing medication administration systems, and/or automated dispensing machines to prompt practitioners to review the salt formulation and indication for all penicillin G orders.
Organizational Leadership
- Share this bulletin with staff and prescribers to raise awareness of the risk of mix-ups with penicillin G salt formulations and the potential for patient harm.
Community Care Providers (e.g., Public Health Units)
- Educate patients with a diagnosis of syphilis that they can expect 2 IM injections for a single treatment/dose. If the patient reports receiving only 1 injection, check with hospital staff to verify that the correct product and dose were administered.
- If follow-up identifies a patient with syphilis who is not responding to treatment, despite having received penicillin therapy, consider a medication error as part of the differential diagnosis.
All health care providers and other stakeholders are encouraged to report suspected medication errors involving penicillin mix-ups to https://www.ismpcanada.org/err_ipr.htm for continued learning.
CONCLUSION
With syphilis cases on the rise in Canada,1 the use of penicillin G benzathine is increasing. System safeguards are needed to reduce the likelihood of mix-ups between different salt formulations of penicillin G. Hospitals, health care providers, and public health units involved in treating and monitoring patients with syphilis are urged to review their practices and help implement system enhancements to ensure safe and appropriate prescribing, dispensing, and administration of penicillin G benzathine.
Sponsors
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
To receive ISMP Canada Safety Bulletins and Newsletters visit: https://ismpcanada.ca/safety-bulletins/#footer
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2024 Institute for Safe Medication Practices Canada.
