INTRODUCTION
ISMP Canada has analyzed several reports* describing administration of an incorrect respiratory syncytial virus (RSV) immunizing agent to patients. These reports have described infants receiving an adult vaccine, pregnant persons receiving the incorrect adult vaccine, and mix-ups with other vaccines. Key contributing factors included lack of familiarity with the numerous new RSV immunizing agents (Table 1), poor design of vaccine storage areas, and lack of labelling of pre-drawn syringes. Learning is shared here to inform continuous improvement activities that support safe administration of the right product to the right patient.
TABLE 1. Respiratory Syncytial Virus (RSV) Immunizing Agents Marketed in Canada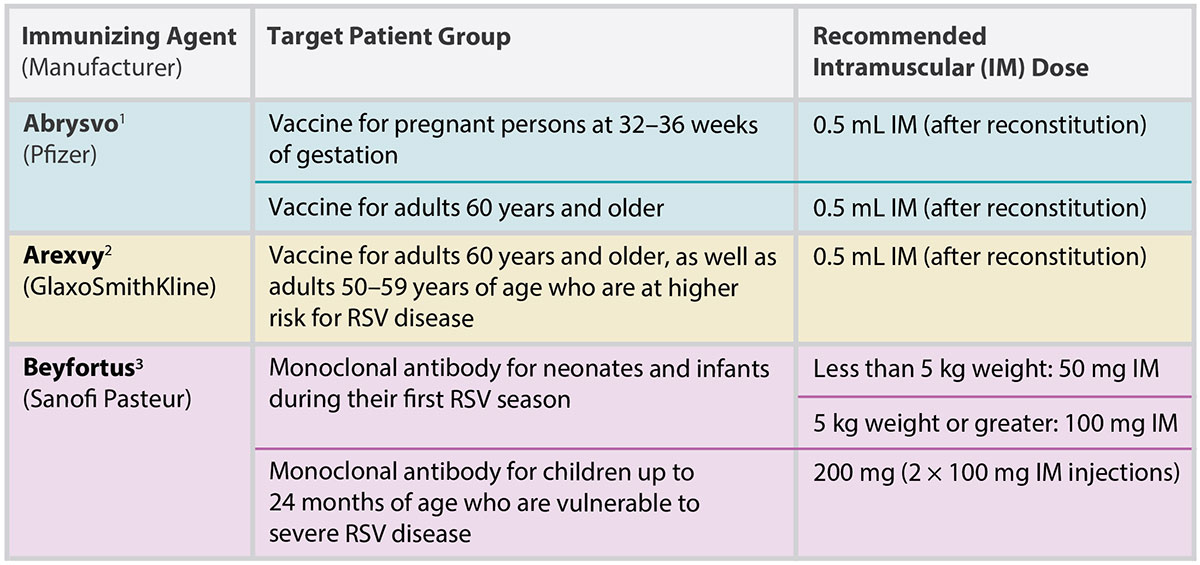
RECOMMENDATIONS
- Incorporate distinguishing features of each product in the electronic drug file (e.g., Beyfortus – monoclonal antibody – pediatric use up to 24 months of age) to support correct selection in electronic drop-down menus.
- Optimize storage areas4 to enable distinction among various products (e.g., use a separate shelf for the pediatric product). Display a quick reference guide in the storage area to support correct product selection.
- Before administration, ask the patient and/or caregiver to confirm the expected immunization.4 Verbalize the product name and indication for the immunization (e.g., Abrysvo to prevent RSV infection in pregnant individuals) and consider showing the vial or labelled syringe to the patient as a second check.
- Administer the immunizing agent immediately after preparation. If this is not possible, label each pre-drawn syringe5 according to jurisdictional regulations (e.g., product name, dose/volume, beyond-use date,4 and patient name).
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
