INTRODUCTION
A child’s death resulting from a compounding error, described in an ISMP Canada Safety Bulletin,1 has reinforced the need for a focus on safety in pharmacy compounding. Recommendations set out in that bulletin included the use of unique chemical identifiers and automated identification (e.g., bar codes) for ingredients used in compounding.
ISMP Canada and HealthPRO Procurement Services Inc. partnered to lead an initiative2,3 to improve the labelling of repackaged active pharmaceutical ingredients (APIs). They worked with an API Advisory Panel* that included regulators, API repackagers, community and hospital pharmacists, and compounding associations from across Canada. This bulletin shares recommendations for safer API labels, as well as examples to illustrate improved label content and design.
BACKGROUND
When medications are not commercially available, a pharmacy may be able to prepare compounded products for patients. Compounding may involve the use of APIs. Many APIs are imported by manufacturers in bulk quantities and then packaged into smaller containers by repackagers for subsequent use by pharmacies (Figure 1).

FIGURE 1. Infographic depicting the “journey” of an active pharmaceutical ingredient (API).
There is limited direction on safe label design for repackaged APIs, unlike the guidance provided by the Good Label and Package Practices Guides for prescription drugs, natural health products, and non-prescription drugs.4,5 Many decisions about label content and design are left to the quality control and marketing departments of the repackager. Although repackagers work with their partners to make improvements to labels, there is consensus that good label and package practices for APIs are needed to facilitate improvements. At the same time, it is recognized that better labelling is only one step towards improved safety and is not sufficient on its own to prevent harmful errors.6
SAFE LABELLING DESIGN CONSIDERATIONS
The API Advisory Panel reached consensus on a set of safe labelling design considerations (Box 1) for repackaged APIs, which are complementary to the requirements set out by Health Canada (GUI-0104)7 and the Workplace Hazardous Materials Information System (WHMIS). Examples of leading work to improve labels are presented in alphabetical order by company name in Figures 2, 3, and 4.


FIGURE 2. Sample label displaying select safe labelling design considerations A, B, C, D, E, F, I, J from Box 1.
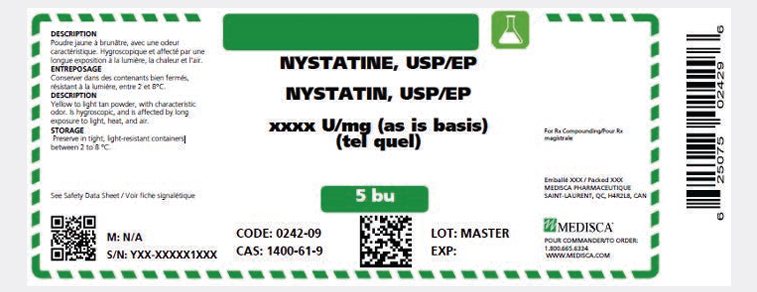
FIGURE 3. Sample label displaying select safe labelling considerations A, C, D, E, I, J from Box 1.

FIGURE 4. Sample label displaying select safe labelling considerations A, D, E, F, J from Box 1.
FOLLOW UP ACTIONS TO DATE
Integrating use of the Chemical Abstracts Service (CAS) Registry Number into practice and workflow are examples of knowledge transfer efforts to enhance safety.
- HealthPRO added the CAS number to its online contracting criteria and published an article describing its work to advance safety. This was done to encourage the use of unique CAS numbers on product labels and improve clarity in the contracting process.9
- In 2021, the Ontario College of Pharmacists published an article about the potential for unique CAS numbers to reduce medication errors.10
- The National Association of Pharmacy Regulatory Authorities (NAPRA) has included the CAS number as an example of a unique identifier in its Master Formulation Record template.11
- A Canadian health care organization recently shared that they have integrated the CAS number into the drug description record used in their electronic system for dispensing and compounding.
Safety bulletins have continued to raise awareness of the risks associated with compounding and the opportunities for continued improvement.12,13 A newsletter for consumers provided tips for parents whose children need medications that must be compounded.14
CONCLUSION
Next steps include knowledge dissemination and knowledge translation to continue to advance safe labelling for repackaged APIs and integrate safety measures into compounding practices. ISMP Canada is supporting a multistakeholder approach to increasing safety for patients who need compounded medications. Interested parties are welcome to join the collaborative efforts by contacting info@ismpcanada.ca.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
