When the Antidote Causes Harm: Preventing Errors with Intravenous Acetylcysteine
Approximately 4500 Canadians are hospitalized each year because of acetaminophen overdose. Although intravenous (IV) infusion has been widely accepted as a safe mode of administration for acetylcysteine, serious errors have occurred, leading to life-threatening conditions and/or death.
KEY POINTS
- Acetylcysteine infusion overdose refers to 1 of 3 scenarios: (1) overdose of intravenous (IV) acetylcysteine on a milligram per kilogram (mg/kg) basis, (2) use of an excessive amount of IV fluid to administer the acetylcysteine; or (3) a combination of scenarios 1 and 2. All scenarios are potentially life-threatening.
- Early warning signs and symptoms of acetylcysteine infusion overdose may include confusion, irritability, restlessness, headache, and/or intractable vomiting.
- Patient harm may also include hemolytic uremic syndrome, seizures, cerebral edema, and brain herniation.
- Lower-weight patients, such as young children (and possibly lower-weight adolescents), are especially vulnerable. For this group, re-examine regimens that use 1-litre D5W infusion solutions to administer acetylcysteine, and consider use of weight-based infusion volume limits.
- Hospitals/health regions should formalize a standardized order set that specifies the dose, type and volume of infusion solution, and the infusion rate or duration, as well as the applicable monitoring parameters. Health care providers (including prescriber, nurse, pharmacist, poison centre staff) should have, for each patient, a shared understanding of the prescribed acetylcysteine dose, dilution, rate, duration, and potential overdose symptoms. In addition, patients and families should understand the care plan and be encouraged to ask questions and share their concerns.
- ISMP Canada has received 3 reports of death or severe harm related to one type of error: continuation of the loading dose IV infusion rate instead of reduction to a lower rate for the maintenance dose, resulting in a 10-fold dose error. Use of a 1-bag, single-concentration regimen was described in all 3 reports.
- Do not administer the loading dose from an IV bag containing the maintenance dose unless the smart infusion pump has the option to program and automatically switch from delivery of the loading dose to delivery of the maintenance infusion, with a separate dose limit for each. If such a pump is not available, prepare a separate bag for the loading dose and maintenance dose infusions.1
- The complexity associated with acetylcysteine administration is partly related to translation of guidance from poison centres to local standardized order sets. Numerous regimens are used by poison centres across Canada, and this variation contributes to the risk of errors. For this and other reasons described in the bulletin, ordering or prescribing “NAC per protocol” is not recommended.
- ISMP Canada does not endorse a specific regimen at this time and calls upon applicable experts from across the country to collaboratively develop a panCanadian protocol to support a consistent approach to the safe treatment of acetaminophen overdoses.
- Analysis of acetylcysteine infusion errors also identied a number of other contributing factors and recommendations that can be locally implemented to improve safety in the short term. See Table 2 for a complete summary.
INTRODUCTION
Approximately 4500 Canadians are hospitalized each year because of acetaminophen overdose.2 Acetylcysteine (also referred to as N-acetylcysteine or NAC) is effective as an antidote for acetaminophen poisoning3 and indicated to prevent or lessen hepatic injury which may occur following the ingestion of a potentially hepatotoxic quantity of acetaminophen.4 Although intravenous (IV) infusion has been widely accepted as a safe mode of administration for acetylcysteine, serious errors have occurred, leading to life-threatening conditions and/or death.5-7
Between 2020 and 2022, ISMP Canada received 2 reports of fatal acetylcysteine infusion overdose incidents involving Canadian patients. An ISMP Canada Safety Bulletin was published shortly after the second event, alerting stakeholders to the potentially lethal consequences of acetylcysteine infusion errors.8 ISMP Canada has learned of additional reports of such errors and undertook, in collaboration with various stakeholders, an analysis of a cluster of reported cases. Key findings from the analysis are shared in this bulletin, along with safety improvement recommendations to reduce the risk of patient harm and death from acetylcysteine infusion errors.
BACKGROUND
Acetylcysteine Infusion Overdose
Acetylcysteine infusion overdose refers to 1 of 3 possibilities:
- Overdose of IV acetylcysteine on a milligram per kilogram (mg/kg) basis
- Case reports in the literature suggest that acetylcysteine overdose is associated with cerebrotoxicity, hemolytic uremic syndrome, and anaphylactoid reactions. Overdoses have resulted in vomiting, seizures, cerebral edema, and/or death.5-7,9-12
- Use of an excessive amount of IV fluid to administer the acetylcysteine
- Effects of over-administration of fluid during acetylcysteine administration can vary with the type of fluid used and the infusion rate and are highly dependent upon patient characteristics such as weight and comorbidities.
- Harm from fluid overload is of particular concern in low-weight pediatric patients.13-15
- Hyponatremia caused by hypotonic fluids (e.g., dextrose 5% in water [D5W], which becomes hypotonic when infused) is a concern with over-infusion of fluids and has clinical features that overlap with those of acetylcysteine toxicity.
- A combination of scenarios 1 and 2.
ACETYLCYSTEINE INFUSION REGIMENS
Currently, there are multiple approaches to the use of acetylcysteine for acetaminophen overdose across Canada, including various regimens (as part of specific protocols) available from poison centres and hospitals/health regions. ISMP Canada does not endorse a specific regimen at this time. Differences among regimens may relate to the number of bags prepared and steps involved, loading dose calculations, drug concentrations, infusion volumes, types of IV fluid, terminology, criteria for starting and stopping an infusion, and parameters for monitoring the patient during infusions. Boxes 1 to 3 describe various acetylcysteine infusion regimens used in Canada, including those publicly available16-22 as well as examples shared by a number of hospitals.
Errors describing harm after IV administration of acetylcysteine have been reported with use of various regimens.5-7,23 Vulnerabilities in common include:
- use of incorrect patient weight
- miscalculation of intended dose
- errors in preparation
- pump programming errors (e.g., repeated programming of the loading dose)
- deficiencies in patient monitoring (e.g., lack of recognition of the signs and symptoms of hyponatremia and/or acetylcysteine toxicity), and
- risks introduced when transferring a patient between facilities who may not share a common protocol or compatible smart pumps.
Importantly, each acetylcysteine infusion regimen also has unique vulnerabilities with the potential to contribute to errors.
3-Bag (3-step, 3-Infusion) Regimen
Historically across Canada, acetylcysteine was administered using a 3-bag regimen (also known as a 3-step or 3-infusion regimen), consisting of separate bags for a loading dose and maintenance doses.4,24 The complexity of the 3-bag regimen and related dosing errors have been documented in the literature, including interruptions in therapy.25 The risk and severity of adverse reactions, notably anaphylactoid reactions, have been associated with the initial rate of acetylcysteine infusion. To address this, most regimens now involve administering the loading dose over 1 hour or longer.26
BOX 1. Three-bag (3-step) regimen delivering 300 mg/kg over 21 h16
Bag 1: Loading dose of 150 mg/kg over 60 min (1 h)
Bag 2: Infusion of 50 mg/kg dose over 4 h
Bag 3: Infusion of 100 mg/kg dose over 16 h
1-Bag (2 Step, 2 Infusion rates) Regimen
Since 2019, several provincial poison centres in Canada have developed and adopted a 1-bag, 2-step regimen for which a single concentration is used for both the loading and maintenance doses. A potential benefit of a 1-bag regimen is the reduction in the number of steps for preparation and administration processes and the associated reduction in omission errors and interruptions in therapy.27-29 A key risk with the use of the 1-bag regimen is inadvertent continuation of the bolus rate to administer the maintenance dose; ISMP Canada has received incident reports of fatal acetylcysteine infusion overdoses associated with this type of error. This risk is related to the total amount of drug and total fluid volume available in a 1-bag single concentration regimen, with the resulting potential to cause harm, particularly when patients are low weight.
BOX 2. One-bag (2-step) single-concentration* regimen
- Loading dose of 150 mg/kg for 1 h and maintenance dose of 15 mg/kg/h for 20 h (total of 450 mg/kg over 21 h)17
or - Loading dose of 60 mg/kg/h for 4 h and maintenance dose of 6 mg/kg/h until advised to stop by poison centre or until stopping criteria are met18
*Concentration of 30 mg/mL17 or 38.7 mg/mL22 (40 mg/mL or 50 mg/mL for fluid-restricted patients)
2-Bag (2-step, 2-Infusion) Regimen
As shown in Box 3, some hospitals/health regions in Canada have moved to variations of a 2-bag regimen.
Of interest, a 2021 report from CADTH (Canadian Agency for Drugs and Technologies in Health) notes that 2 evidence-based guidelines recommend a 2-bag regimen for acetylcysteine, consisting of a loading dose and a maintenance dose.30 This regimen consists of a weight-based loading dose (mg/kg) administered over 4 hours, a weight-based maintenance dose (mg/kg) administered over 16 hours in dextrose 5% (D5W) or sodium chloride 0.9% (noting compatibility with 0.45% saline + 5% dextrose) , and weight-based limits for admixture fluid volumes (mL/kg).31 In Australia and New Zealand, this 2-bag (2-step) regimen is widely used.30
The complexity associated with acetylcysteine regimens is partly related to translation of guidance from the applicable poison centre into local procedures such as standard order sets, computerized prescriber order entry and clinical decision supports, hospital monographs, and/or infusion pump processes and drug libraries. User testing, clinical education and change management strategies at the level of local hospitals/health regions vary significantly and may not incorporate human factors considerations and real-time clinical checks and supports.
BOX 3. Two-bag (2-step) regimen
- Two separate bags containing the same concentration of drug {first bag is delivered as a weight-based dose [mg/kg/h] for 1 or 4 hours, with remainder in bag discarded; second bag delivers a weight-based maintenance dose [mg/kg/h])
or - Two separate bags, the first containing a weight-based loading dose (mg/kg) in a smaller volume of infusion solution to be administered over a defined period and the second delivering a weight-based maintenance dose (mg/kg/h) from a solution with standard concentration.
ANALYSIS METHODOLOGY
Reported Medication Incidents
Voluntary reports of medication incidents related to IV administration of acetylcysteine in the hospital setting were extracted from 3 ISMP Canada reporting databases (National Incident Data Repository for Community Pharmacies, Individual Practitioner Reporting, and Consumer Reporting) and the Canadian Institute for Health Information’s National System for Incident Reporting (NSIR) database* for the 5-year period from August 1, 2017, to July 31, 2022,† using the following search terms: ‘*acetylcysteine’, Acetylcysteine, NAC, Mucomyst, Mucosil, Acetadote, Parvolex. The analysis was conducted according to the Canadian Incident Analysis Framework32 methodologies for comprehensive and multi-incident analyses.
Searches of the Canada Vigilance Program and medical device incident reporting databases and of the US Manufacturer and User Facility Device Experience (MAUDE) database, using the same dates and the keyword “acetylcysteine”, were also completed. As well, an international call for reports of medication incidents related to acetylcysteine occurring within the same period was disseminated by email through the International Medication Safety Network.
Literature Search and Resource Review
A literature synthesis was completed using the MEDLINE, Embase, PubMed, and CINAHL databases. The search terms “acetylcysteine”, “drug overdose”, “medication errors”, “adverse events”, “smart pump”, “user interface”, and “IV administration protocol” were used to identify articles written in English and published between September 2017 and September 2022.
In addition, provincial poison centres and selected hospitals/health regions were asked to provide copies of their protocols, standardized order sets (including pump instructions), manufacturer product monographs, and hospital-specific monographs. Various acetylcysteine infusion protocols used by selected health care organizations and provincial poison centres across all Canadian provinces and territories were analyzed.
The acetylcysteine manufacturer product monograph in Canada4 provides a dosage and preparation guide for the 3-bag (3-step) regimen and includes weight-adjusted infusion volumes for the first dose provided over 15 minutes, use of a 500 mL D5W bag for the second infusion, and a 1-litre D5W infusion bag for the third infusion. It notes:
- Intravenous administration of acetylcysteine can cause fluid overload, potentially resulting in hyponatremia, seizure and death.
- Use with caution in children, patients requiring fluid restriction or those who weigh less than 40 kg because of the risk of fluid overload. The volumes and rates of infusion for children suggested must be adjusted according to the medical circumstances.
- Restrictions in the volumes of parenteral fluids administered and the state of hydration and serum electrolytes for each patient must be monitored closely.4
The package insert for the US FDA approved product provides a dosage and preparation guide for the 3-bag (3-step) regimen, with the first infusion delivered over an hour. Infusion volumes are defined, and limited based on weight, for patients less than 40 kg.13
Interviews with Key Informants
Interviews were held with health care providers and quality/safety staff from hospitals/health regions, toxicologists and other staff at poison centres, and coroners. These interviews led to identification of 2 additional case reports, published in 199714 and 2015,15 respectively, that described hyponatremia induced by acetylcysteine infusion. The case reports described toddlers who experienced seizures that were attributed to infusion of large volumes of D5W, which resulted in excess free water.
A multidisciplinary team conducted a site visit to a tertiary hospital to learn about clinical decision support documentation and to review the hospital’s infusion pump from a human factors perspective, using an example order and the hospital’s monograph for acetylcysteine.
QUANTITATIVE ANALYSIS
Canadian database searches yielded a total of 79 cases that were screened for inclusion (Table 1). Of these, 61 were included in the quantitative and qualitative analyses.‡ Duplicate or incomplete reports were excluded, as were reports unrelated to the IV administration of acetylcysteine. Figure 1 depicts the breakdown of near miss/no harm and harm incidents.
Among the reports indicating patient harm were descriptions of 4 deaths related to acetylcysteine infusion overdose (see Box 4 for more details). These reports described rates of infusion 6 to 16 times higher than intended. Three of these deaths involved pediatric patients (a toddler and 2 teenagers). The reported effects of acetylcysteine infusion overdose included intractable vomiting during the infusion, clinical deterioration with seizures, severe brain injury due to cerebral edema, and/or brain herniation that progressed to brain death.
Reports of incidents related to acetylcysteine infusion found in the MAUDE database and other international cases were reviewed. Analysis findings from these international reports were consistent with the analysis findings from the Canadian reports.
The stages of the medication-use process involved in the incidents were administration (for more than half of the cases), specifically pertaining to over-infusion and omission of doses; prescribing (e.g., order documentation); preparation and dispensing; and monitoring.
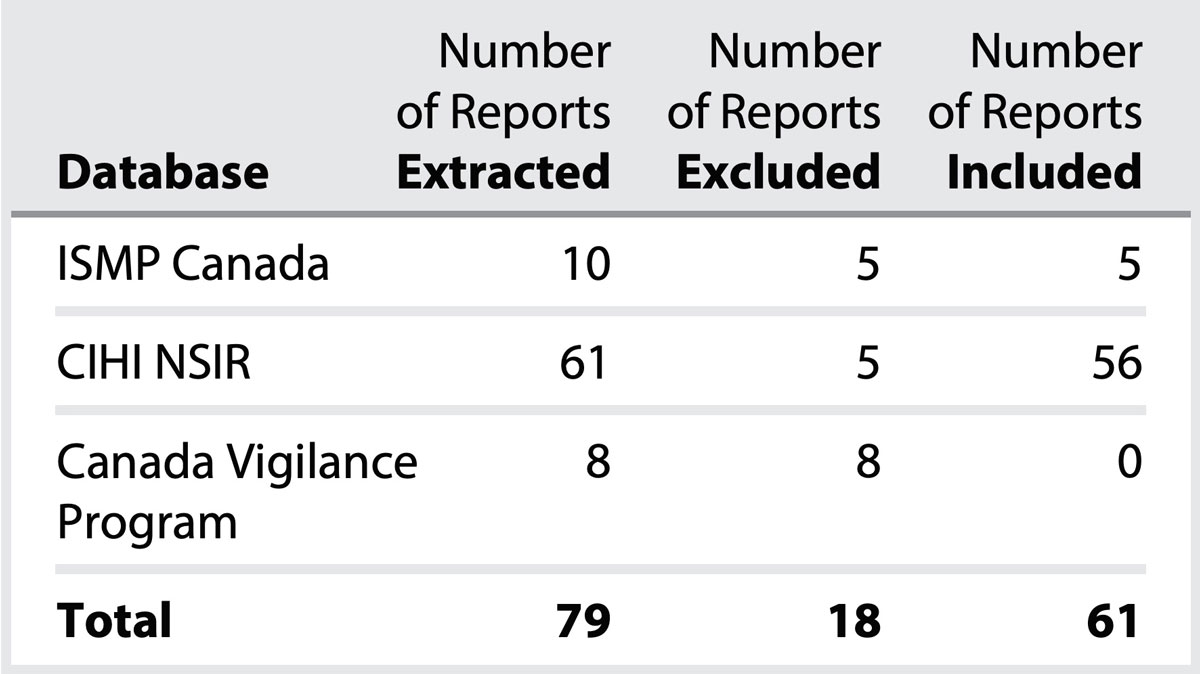
TABLE 1. Results of Database Searches

FIGURE 1. Distribution of Incident Outcomes (n = 61)
BOX 4. Database Reports Associated with Fatal Acetylcysteine Infusion Overdoses
Fatal incidents (n = 4) related to acetylcysteine infusion overdose involved the following errors:
- administration of the maintenance infusion using the infusion rate of the loading dose (10-fold error), with both doses prepared in the same 1-litre infusion bag of dextrose 5% in water (D5W)
- miscommunication/confusion about the regimen the patient was to receive (e.g., confusing the 3-step “mg/kg” dose and the 2-step “mg/kg/h” dose)
- miscalculation of a concentration or rate of infusion
QUALITATIVE ANALYSIS
Key qualitative findings identified in the ISMP Canada analysis are summarized in Table 2, including contributing factors and applicable recommendations.
QUALITATIVE ANALYSIS - Key Finding
Design of Acetylcysteine Protocol and Standardized Order Set; Availability of Resources
Contributing Factor:
Inconsistent and/or unclear terminology in some poison centre protocols and/or hospital/health region standardized order sets increases risk of errors.
Recommendations for Poison Centres:
- Collaboratively develop a pan-Canadian protocol to support a consistent
approach to the safe treatment of acetaminophen overdoses. - Incorporate a step in the patient care consultation process to request the hospital
or health region’s standardized order set and to confirm mutual understanding of
the dose being delivered and next steps.
Recommendations for Hospitals and Health Regions:
- Develop standardized order sets according to the following principles:
- With support from an interdisciplinary team, use clear and concise directions and consistent terminology and avoid potentially dangerous abbreviations.
- Where applicable, incorporate poison centre protocol and guidance into order sets. For pediatric patients, also consider local pediatric centre guidance.
- Ensure consistency with the organization’s infusion pump programming terminology and sequence of programming steps.
- Include direction for when to contact the local provincial poison centre (https://infopoison.ca/) and steps to access a medical toxicologist and pharmacist for consultation, when applicable.
- Incorporate a step in the poison centre consultation process, to provide the hospital/health region’s standardized order set and to confirm mutual understanding of the dose being delivered and next steps.
- Include dosing tables to provide calculation supports for preparing doses and/or checking the rate/duration of infusions.
- Check that smart pump drug libraries include acetylcysteine, with applicable parameters established according to specific directions provided in the standardized order set.
Contributing Factor:
Use of a 1-litre bag of dextrose 5% in water (D5W) for administration of acetylcysteine in lower-weight patients (e.g., toddlers) increases potential for harm.
Recommendations for Poison Centres, Hospitals, and Health Regions:
- Develop standardized protocols and standardized order sets according to the following principles:
- Clearly differentiate pediatric weight-based volume and infusion directions from instructions for adult patients, to reduce the risk of volume overload and/or overdose.
- Ensure that the intravenous (IV) bag size used to prepare the acetylcysteine dose is appropriate for lower-weight patients, who are at increased risk of harm from fluid overload.
- Include monitoring parameters for electrolytes and fluid balance.
- Include monitoring parameters for early signs of infusion overdose (e.g., headache, confusion, nausea and vomiting, irritability, disorientation, decreased level of consciousness, seizures).
- Additional notes:
- Acetylcysteine is compatible with D5W, 0.9% saline, 0.45% saline, and 0.45% saline + 5% dextrose solutions.13,31,33
- Re-examine regimens that use 1-litre D5W infusion solutions to administer acetylcysteine in this population; consider use of weight-based infusion volume limits. Consider the osmolarity, tonicity, and total volume of the IV solution (e.g., D5W becomes hypotonic upon infusion).
Contributing Factor:
Lack of user testing of protocols and/or standardized order sets with local prescribers, pharmacists, and nursing staff before implementing a change in the protocols and/or standardized order sets reduces the likelihood that vulnerabilities will be detected and addressed before implementation.
Recommendations for Hospitals and Health Regions:
- Complete the following steps before finalizing and implementing a standardized order set for IV administration of acetylcysteine:
- User testing of the order set.
- Hospital/health region risk assessment, with considerations that include access to real-time pharmacist support, and also the potential for patient transfer to other facilities (with different protocols/standardized order sets).
- Consider the following additional strategies:
- Where available, consider testing order sets and protocols in a simulation laboratory.
- Develop a change management approach, including education strategies, for prescribers, pharmacists, and nurses before launching new or changed standardized order sets and/or other clinical supports.
Contributing Factor:
Having out-of-date, confusing, or conflicting clinical resources accessible to health care providers increases the likelihood of errors in prescribing, preparing, and/or administering the infusion.
Recommendations for Hospitals and Health Regions:
- Ensure that the up-to-date poison centre protocol and hospital/health region standardized order set (and other related supports) are readily accessible to any prescriber, pharmacist, or nurse who may need it.
- Remove outdated or conflicting resources from patient care areas.
QUALITATIVE ANALYSIS - Key Finding
Canadian Product Monograph
Contributing Factor:
The Canadian Product Monograph(s) do not reflect leading practices (e.g., for the initial infusion rate, and guidance for determining weight-based infusion volume limits), increasing the risk of errors.
Recommendations for Product Manufacturers (Sponsors)
- Review and update product monographs based on findings and recommendations in this Safety Bulletin.
QUALITATIVE ANALYSIS - Key Finding
Decision to Treat and Prescribing of Acetylcysteine
Contributing Factor:
Lack of real-time access to clinical “decision-to-treat” resources for acetylcysteine infusion increases the likelihood that treatment will be provided when it is not indicated.
Recommendations for Hospitals and Health Regions:
- Provide prescribers with real-time access to clinical “decision-to-treat” and prescribing resources (e.g., clinical pathways including a nomogram, poison centre contact information), as well as access to laboratory resources.34
Contributing Factor:
Prescribing acetylcysteine using the order or phrase “as per protocol” without understanding the implications of dosing and fluids administered increases the risk of errors in preparation, administration, and monitoring.
Recommendations for Prescribers:
- Use a standardized order set to reduce the risk of errors associated with IV administration of acetylcysteine and to ensure a mutual understanding among care team members (including prescriber, nurse, pharmacist, poison centre staff, patient, and family) of how acetylcysteine is being provided and monitored.
- Avoid using verbal or telephone orders which may lack clarity or be misinterpreted.
QUALITATIVE ANALYSIS - Key Finding
Preparation and Administration of Acetylcysteine for Infusion
Contributing Factor:
Having nurses prepare the infusion bag(s) without the benefit of pharmacist order verification and preparation reduces the likelihood that a dosing error will be detected and prevented.
Recommendations for Hospitals and Health Regions:
- Ensure that clear instructions for preparation and administration of acetylcysteine infusion are readily available.
- Consider having the pharmacy prepare the IV bag for maintenance acetylcysteine doses, and possibly the loading dose, if this can be done in a timely fashion.
- Implement an independent double-check process for determining/verifying doses and dose preparation.
Contributing Factor:
Inability of nurses to easily and quickly check to confirm doses/rates for an acetylcysteine infusion reduces the likelihood that an error will be detected and prevented.
Recommendations for Hospitals and Health Regions:
- Add clinical advisories that appear during infusion pump programming to help guide a check of the pump settings.
- Designate acetylcysteine as a high-alert medication requiring an independent double check when initiating and adjusting IV pump settings.
- Provide initial and ongoing education to the nursing team who will manage delivery of medications via pumps.
Contributing Factor:
Transfer of care between staff (e.g., covering breaks, moving between units) and between facilities (e.g., with potentially different protocols) without mutual understanding of the acetylcysteine treatment in progress increases the risk of errors.
Recommendations for Nurses:
- At any transfer of care,
- assess the prescriber’s order, the step of the standardized order set in progress (i.e., loading or maintenance dose), and ensure that IV infusion dose rate/duration has been programmed correctly,
- assess that infusion pumps and equipment at the receiving facility are compatible with the standardized order set used, and
- ensure a mutual understanding of the key monitoring parameters.
QUALITATIVE ANALYSIS - Key Finding
Infusion Pump Programming
Contributing Factor:
Choosing “no drug selected”, “other drug”, “drug X”, “basic mode”, or a generic “IV fluids” setting (depending on the pump model) bypasses a smart pump’s drug error reduction software and reduces the likelihood that a programming error will be detected and prevented.
Recommendations for Hospitals and Health Regions:
- Include acetylcysteine in infusion pump drug libraries and build specific regimens for adults and pediatric patients.
- Develop a timely process to support nurses when incorrect or missing drug library settings are identified, as well as a clear process to remedy the concern.
- Review instances of “no drug selected” and pump override reports to identify quality improvement opportunities.35
Contributing Factor:
Delivery of a loading dose and maintenance dose from the same infusion bag increases the risk of errors.
- Use of pumps that allow the dose to be programmed repeatedly (e.g., with a “continue” option) increases the risk if a one-bag (2-step, 2-infusion rates) single-concentration regimen is used.
Recommendations for Hospitals and Health Regions:
- Do not administer loading doses from the IV bag containing the maintenance dose if the smart infusion pump does not have the option to program and automatically switch from delivery of the loading/bolus dose to delivery of the continuous maintenance infusion with a separate dose limit for each. If such pumps are not available prepare a separate bag for the loading dose and maintenance dose infusions.1
- Design the standardized order set to ensure consistent guidance for the administration of acetylcysteine in all areas to support the ability to safely transfer and care for patients.
Recommendations for Poison Centres:
- Design a pan-Canadian protocol that can be safely implemented regardless of the type of infusion pump available in the hospital or health region.
Contributing Factor:
Delays in IV infusion pump upgrades may increase the risk of errors.
Recommendations for Hospitals and Health Regions:
- Prioritize IV infusion pump upgrades that are designed to include additional safety features.
QUALITATIVE ANALYSIS - Key Finding
Monitoring of Acetylcysteine Infusions
Contributing Factor:
Lack of timely recognition by physicians and nurses of the patient’s signs and symptoms associated with acetylcysteine infusion overdose reduces the likelihood that an infusion overdose will be detected and appropriate interventions provided.
Recommendations for Care Teams:
- Ensure that patient monitoring includes assessment for signs and symptoms of concern and actions to be taken if they occur.
- Signs and symptoms of acetylcysteine infusion overdose occur early and may include various symptoms that are also associated with hyponatremia, such as confusion, irritability, restlessness, headache, intractable vomiting (despite treatment with an antiemetic such as ondansetron), altered level of consciousness, and/or seizure.
- Ensure laboratory testing that captures indices relevant to acetylcysteine therapy and fluid management are performed according to the standardized order set, and review results promptly to ensure patient treatment is progressing as anticipated and/or to identify concerns.
- If over-infusion is suspected, contact the local poison centre to speak with a toxicologist, and seek expertise in fluid management.
QUALITATIVE ANALYSIS - Key Finding
Patient and Family Engagement
Contributing Factor:
Lack of patient/family engagement in the treatment plan, including monitoring parameters, reduces the likelihood that errors will be detected and shared with health care providers.
Recommendations for Hospitals and Health Regions:
- Engage the patient/family in the plan of care, including the plan for loading and maintenance dose infusions with anticipated timelines. During discussion, describe signs and symptoms of concern to bring to the attention of a health care provider, including confusion, irritability, restlessness, and intractable vomiting.
CONCLUSION
An analysis of reported incidents involving acetylcysteine in treating acetaminophen overdose identified the variety, complexity, and vulnerabilities for error in existing poison centre and hospital/health region treatment regimens in Canada. ISMP Canada does not endorse a specific regimen at this time and calls upon applicable experts from across the country to collaboratively develop a pan-Canadian protocol for the safe treatment of acetaminophen overdoses. There are a number of recommendations provided in this bulletin that can be locally implemented to improve safety in the short term.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
