INTRODUCTION
Paxlovid (a co-packaged product containing nirmatrelvir and ritonavir tablets) was recently approved by Health Canada for the treatment of mild to moderate COVID-19 in adults at high risk for progression to severe disease.1 Several key safety considerations have been identified: (1) the need for product manipulation before it is dispensed for use by patients with moderate renal dysfunction, (2) the display of critical information (e.g., dosing) about the co-packaged product in prescribing and other medication software, and (3) the need for updated drug interaction checking software.
To enable rapid access in Canada, Pfizer is providing the US product labelled for emergency use.2 Products meeting Canadian label requirements (e.g., bilingual, drug identification number) will be made available as soon as possible. One box of Paxlovid contains 5 blister cards (for a typical 5-day treatment). Each card provides 2 doses to be administered in a single day, with each dose consisting of 2 (pink) nirmatrelvir 150 mg tablets and 1 (white) ritonavir 100 mg tablet.1
NEED FOR PRODUCT MANIPULATION FOR PATIENTS WITH MODERATE RENAL IMPAIRMENT
When Paxlovid is dispensed for this patient population, 1 tablet of nirmatrelvir is to be removed from the morning dose and 1 tablet of nirmatrelvir from the evening dose (see red circles in Figure 1). This step is required to dispense the recommended reduced dose of nirmatrelvir 150 mg (i.e., 1 tablet) twice daily.1-3
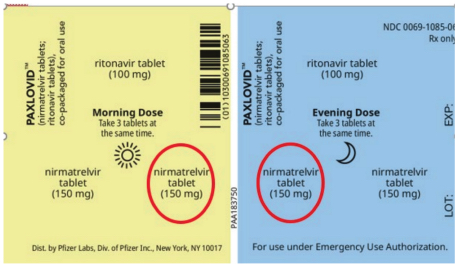
Figure 1. US Emergency Use Paxlovid blister label currently available in Canada.3
ISMP (US) recently described incorrect dispensing of Paxlovid for a patient with moderate renal dysfunction. The dispensed product was missing ritonavir tablets that were needed, whereas nirmatrelvir tablets were present that should have been removed.4
To support pharmacists dispensing Paxlovid, a province-wide collaboration in Saskatchewan was undertaken. Additional resources were developed, including a patient information handout describing renal dosing and a template to create stickers for the purpose of communicating package manipulation.5
Ensure clear communication with patients if the product has been manipulated to provide a reduced dose.
DISPLAY OF CRITICAL INFORMATION IN MEDICATION SOFTWARE SYSTEMS
A co-packaged medication requiring dose adjustment of one component and not the other present challenges for medication software systems, such as prescriber order entry systems, electronic health or medical records, medical administration records, and pharmacy systems.
Specify the dose for each active ingredient on all prescriptions.2
NEED FOR UPDATED DRUG INTERACTION CHECKING SOFTWARE
Ritonavir interacts with many other medications; such interactions can lead to life-threatening situations.6 Many pharmacies rely upon drug interaction software to identify interactions; however, one hospital reported that their drug interaction software had not yet been updated to detect interactions involving Paxlovid.
Confirm drug interaction software is updated to capture Paxlovid interactions.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
