Opportunities for Safer Management of Fertility Medications: A Multi-Incident Analysis
Infertility affects approximately 1 in 6 people worldwide. This multi-incident analysis of reported incidents involving fertility medications highlights opportunities to reduce the risk of error and to support the safe and timely use of medications to treat infertility and to promote and/or sustain pregnancy.
INTRODUCTION
Infertility affects approximately 1 in 6 people worldwide.1 The underlying reasons for female and male infertility are multifactorial. Treatment options for certain causes of infertility include ovarian stimulation, intrauterine insemination (IUI), and in vitro fertilization (IVF),2,3 each of which involves the use of one or more medications. This bulletin describes the findings of a multi-incident analysis of reports related to fertility medications and shares recommendations to optimize their safe use.

METHODOLOGY
Medication incidents associated with fertility medications, submitted in the 10-year period between January 2015 and January 2025, were extracted from ISMP Canada’s Consumer Reporting program, Individual Practitioner Reporting database, and the National Incident Data Repository for Community Pharmacies (NIDR), as well as the Canadian Institute for Health Information’s (CIHI’s) National System for Incident Reporting (NSIR).*
Key search terms included the generic and brand names of medications commonly used to treat infertility or to promote and/or sustain pregnancy, as well as terms related to fertility or pregnancy (e.g., IVF). Reports of medication use for indications unrelated to fertility were excluded. The multi-incident analysis was conducted according to the methodology outlined in the Canadian Incident Analysis Framework.4
QUANTITATIVE FINDINGS
Of the 694 reports that were retrieved, 258 were included in the analysis.† Most of these incidents were reported as either near misses or causing no harm, while approximately 3% were reported as causing either mild or moderate harm (Figure 1). The most common type of error was reported to be incorrect dose/frequency of the medication, followed by incorrect quantity and incorrect drug (Figure 2).
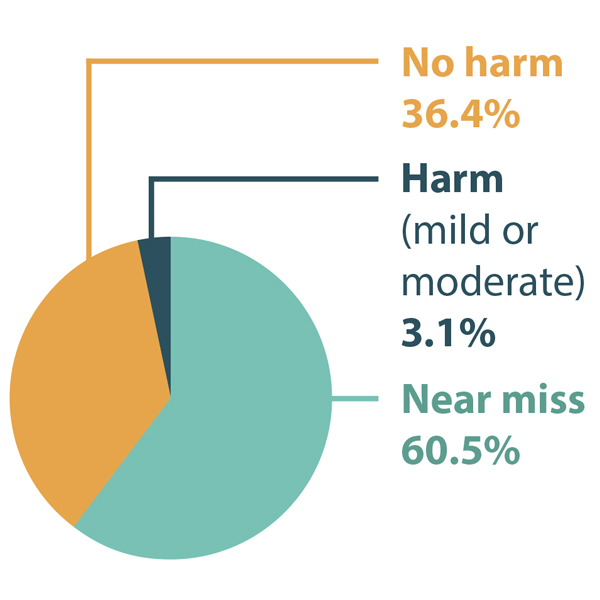
FIGURE 1. Distribution of reported incidents involving fertility medications by level of harm.
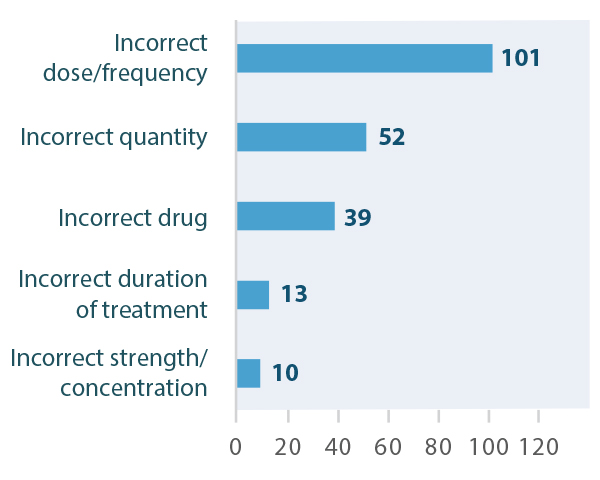
FIGURE 2. Top 5 types of errors reported in incidents involving fertility medications.
QUALITATIVE ANALYSIS
The qualitative analysis of reported incidents involving fertility medications identified 4 main themes (Figure 3).

FIGURE 3. Themes identified in the qualitative analysis of medication incidents involving fertility medications.
THEME: Unique Fertility Dosing Regimens
This theme included incidents in which the medication strength, dose, frequency, route, duration of treatment, and/or quantity were unfamiliar to the pharmacy team. Confirmation bias was a key factor contributing to these incidents, given that the medications may also be used for other indications. For example, the medication described in the incident below (letrozole) can be used for hormone-sensitive breast cancer treatment, in which case it is typically administered daily for years.
Incident Example: A prescription was written for letrozole 5 mg once daily, to be taken on days 3 to 7 of the patient’s cycle. The prescription was for a 3-month supply (i.e., 15 tablets), but the quantity was mistakenly entered as 90 tablets. The pharmacist recognized and corrected the error during clinical verification before the medication was given to the patient.
THEME: Frequent Treatment Modifications
A patient’s fertility journey may entail multiple medications and procedures to treat infertility and promote pregnancy. Medication management differs between the various treatment options (e.g., IUI versus IVF) and regimens may need to be adjusted from one cycle to the next.5 A key factor contributing to the incidents within this theme was the pharmacist copying a previous prescription file without recognizing or incorporating prescribed changes.6 Failure to adjust directions on the label may result in administration errors.
Incident Example: A new prescription for a gonadotropin was brought to the pharmacy. During order entry, a previous prescription was copied, and the directions for use were not changed to reflect the new prescription. As a result, the administration schedule appearing on the label was incorrect. This was identified and corrected before the medications were provided to the patient.
THEME: Time-Sensitive Treatment Regimens
Medications to treat infertility are often needed on specific days of the patient’s menstrual cycle. A missed or delayed dose may mean that the patient must wait for the next menstrual cycle to resume treatment, potentially delaying conception.7 Contributing factors within this theme included miscalculation of the quantity of medication needed, workflow problems (with multiple medications being filled at the same time), and the presence of expired stock in inventory.
Incident Example: A patient had multiple medications to pick up at the pharmacy; however, at the time of pickup, they did not notice that one prescription had not been filled. Over the weekend, the patient called the nurse to report the missing medication. The nurse was able to arrange delivery of the medication directly to the patient, although the patient received the dose later than intended.
THEME: At-Home Medication Administration and Monitoring
Patients may need to self-administer injectable medications7 and self-monitor for signs and symptoms of potential adverse effects or drug interactions. Contributing factors associated with this theme included inadequate training for drug preparation and administration, as well as insufficient explanation of symptoms to watch for and when to seek help.
Incident Example: A patient was prescribed a gonadotropin product; the dose was 150 units daily, to be given subcutaneously. Subsequent testing showed unexpectedly low hormone levels, and further investigation revealed that the patient had been preparing and injecting doses of 75 units for the previous 4 nights. As a result, the IVF cycle had to be cancelled. It was later discovered that the patient had been instructed to use 2 vials of 75 units to generate the desired total dose of 150 units. However, the instructions were misunderstood to mean 1 vial of medication plus 1 vial of diluent, rather than 2 vials of medication.
RECOMMENDATIONS
Prescribers
- Include the indication on the prescription to support the pharmacist’s clinical assessment8 and recognition of the medication’s intended use.
- Consider including a note in the prescription and/or health care record when a change is made to a patient’s fertility medications, especially mid-cycle.
- Review with the patient and provide written instructions describing any changes made to medications mid-cycle.
- Ask the patient to confirm that their pharmacy can provide medications in a timely manner. Medications may not be in stock at the pharmacy at all times and prescriptions may take a few days to fill.
Pharmacy Teams
- Add a note or flag in the patient profile for individuals undergoing fertility treatments. This alerts pharmacy team members to potentially unfamiliar medication doses/schedules.
- With each new prescription, speak with the patient about their current medication regimen, including any changes to their therapy or dose.9
- Ensure that discontinued medications, including previous dosing regimens and logged prescriptions, are inactivated in the patient’s profile.10
- Offer to create a personalized calendar to help the patient document their fertility treatment in each cycle. Include a list of all the medications in that cycle to enable an extra check so that no medications are missed, and scan a copy of the calendar into the patient’s profile.10
- Some clinics allow patient access to a portal through which medication information, including doses and directions for use, is made available. The system sends emails or text messages directly to the patient to communicate reminders or dose changes within their specific treatment plan.
- Provide the patient with the necessary supplies to administer all medications as intended by the physician. For example, provide prefilled syringes where available, split tablets for specific dosing, prepare blister packs for complex regimens, or provide the correct needles to prepare and administer an injection.
All Health Care Providers
- Advise patients about the potential adverse effects of fertility medications, how to manage them, and when to seek urgent care.
- Offer patient resources to support the safe use of fertility medications, such as injection training videos or hands-on training.
- Use the teach-back method to confirm patient understanding of instructions.
- Consider communicating the value of an independent double check when a patient is self-administering their fertility medication (e.g., a double check by their partner).
CONCLUSION
This multi-incident analysis of reported incidents involving fertility medications highlights opportunities to reduce the risk of error and to support the safe and timely use of medications to treat infertility and to promote and/or sustain pregnancy. A key recommendation is to include the indication for treatment on the prescription; this supports pharmacist clinical assessment and provision of patient resources for safe medication preparation and administration.
![]()
The Canadian Medication Incident Reporting and Prevention System (CMIRPS) is a collaborative pan-Canadian program of Health Canada, the Canadian Institute for Health Information (CIHI), the Institute for Safe Medication Practices Canada (ISMP Canada) and Healthcare Excellence Canada (HEC). The goal of CMIRPS is to reduce and prevent harmful medication incidents in Canada.
Funding support provided by Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.

The Healthcare Insurance Reciprocal of Canada (HIROC) provides support for the bulletin and is a member owned expert provider of professional and general liability coverage and risk management support.

The Institute for Safe Medication Practices Canada (ISMP Canada) is an independent national not-for-profit organization committed to the advancement of medication safety in all healthcare settings. ISMP Canada’s mandate includes analyzing medication incidents, making recommendations for the prevention of harmful medication incidents, and facilitating quality improvement initiatives.
Report Medication Incidents (Including near misses)
Online: ismpcanada.ca/report/
Phone: 1-866-544-7672
ISMP Canada strives to ensure confidentiality and security of information received, and respects the wishes of the reporter as to the level of detail to be included in publications.
Stay Informed
Subscribe to the ISMP Canada Safety Bulletins and Newsletters.
This bulletin shares information about safe medication practices, is noncommercial, and is therefore exempt from Canadian anti-spam legislation.
Contact Us
Email: cmirps@ismpcanada.ca
Phone: 1-866-544-7672
©2025 Institute for Safe Medication Practices Canada.
